Combined Genome Wide and Single Nuclei Transcriptomics in Posttransplant Acute Kidney Injury Identifies Early Proinflammatory/Profibrogenic Cell Origin
1University of Maryland, Baltimore, MD, 2Albert Einstein College of Medicine, Montefiore Medical Ctr, Bronx, NY
Meeting: 2022 American Transplant Congress
Abstract number: 278
Keywords: Epithelial cells, Gene expression, Genomics, Kidney transplantation
Topic: Basic Science » Basic Science » 14 - Ischemia Reperfusion
Session Information
Session Name: Ischemia Reperfusion
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:50pm-5:00pm
Presentation Time: 4:50pm-5:00pm
Location: Hynes Room 302
*Purpose: Kidney transplants offer a unique opportunity to evaluate molecular pathways involved in development and response to acute kidney injury (AKI). This prospective study of transplanted kidneys with AKI aims to identify cells involved in injury or impaired reparation.
*Methods: Fifteen AKI biopsies with histological features of AKI (pure-AKI) (collected within 1-month posttransplant) and paired 3-month protocol biopsies (n=30 biopsies) were assayed by microarrays. Normal allografts (NAs) biopsied at 3-months posttransplant were used as controls (n=20). AKI patients were subclassified based on their graft function (high/low) at 36-months posttransplant. A subset of AKI samples (n=4) and NA (n=3) were evaluated using single nuclei (sn) RNA-seq. Using the 10X Genomics Chromium Platform, >40,000 nuclei in Gel-Bead V3 were captured. Analysis of each dataset was generated with the CellRanger software and visualized using UMAP. Distinct cell clusters were identified by their expression of highly variable genes.
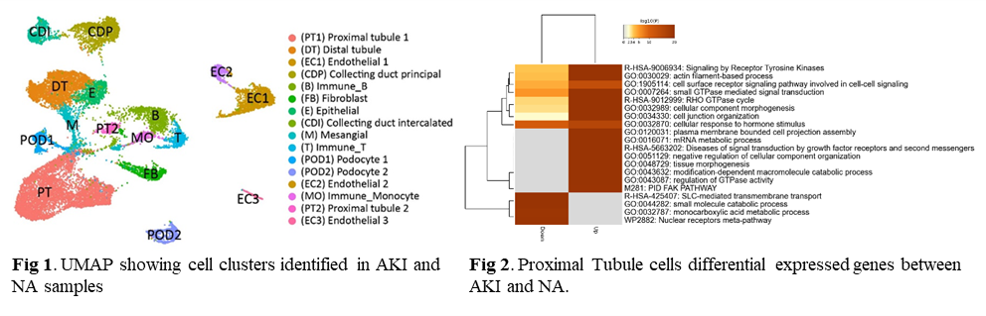
*Results: DEGs (FDR<0.05) between AKI and paired 3-month biopsies showed upregulation of the humoral immune response, neutrophil degranulation, and wound healing in kidney grafts with eventual low function at 36-months posttransplant. Gene enrichment analyses showed upregulation of epithelial mesenchymal transition, angiogenesis, and collagen formation in the same biopsies. Following snRNA-seq, 16 cell clusters were identified from AKI and NA samples. Two types of proximal tubule cells were identified (PT1 and PT2) and characterized by decreased metabolic functions and SLC-mediated transmembrane transport in AKI grafts. PT2 presented a PT injured phenotype with a higher degree of replicative repair. Fibroblasts showed an activated phenotype compared to NAs (extracellular matrix turnover, collagen biosynthesis). Proinflammatory monocytes were predominant in AKI samples.
*Conclusions: DEGs between paired pure-AKI and 3-month biopsies associated with immune activation and impaired reparation in kidney grafts in allografts with low function at 36-months posttransplant. Critically, the analyses of transcriptional profile at single cell resolution identified early proinflammatory/profibrogenic cell subtypes. Further evaluation of cell subtypes and cell-to-cell interactions may lead to the discovery of targeted interventions to avoid progression to fibrosis and graft function loss.
To cite this abstract in AMA style:
McDaniels J, Rousselle T, Bardhi E, Shetty A, Akalin E, Maluf D, Mas V. Combined Genome Wide and Single Nuclei Transcriptomics in Posttransplant Acute Kidney Injury Identifies Early Proinflammatory/Profibrogenic Cell Origin [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/combined-genome-wide-and-single-nuclei-transcriptomics-in-posttransplant-acute-kidney-injury-identifies-early-proinflammatory-profibrogenic-cell-origin/. Accessed July 5, 2025.« Back to 2022 American Transplant Congress