Belatacept Augments Mixed Chimerism in a Novel Nonhuman Primate Kidney Transplant Tolerance Induction Protocol
University of Wisconsin, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 1281
Keywords: Co-stimulation, Mixed chimerism, Primates, Tolerance
Topic: Basic Science » Basic Science » 12 - Immunosuppression & Tolerance: Preclinical & Translational Studies
Session Information
Session Name: Immunosuppression & Tolerance: Preclinical & Translational Studies
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: A novel protocol of antilymphocyte globulin (ATG), tomotherapy-based total lymphoid irradiation (TomoTLI), and adoptive cellular transfer was developed in a preclinical model of MHC-mismatched nonhuman primate (NHP) kidney transplantation. This study sought to provide insight into improved chimerism seen with the addition of costimulation blockade in this mixed chimerism-based tolerance induction model.
*Methods: ATG (4mg/kg x5 doses) with or without TomoTLI (12Gy over 10 fractions) and Belatacept (10mg/kg x4 doses) was applied to a 3-5 MHC antigen mismatched post-kidney transplant rhesus macaque model. Donor hematopoietic cells were infused after induction in the ATG+TomoTLI and ATG+TomoTLI+Bela cohorts. Maintenance immunosuppression consisted of mycophenolate mofetil (15mg/kg/day) and tacrolimus (trough 8-10ng/mL), which was tapered off by week 21 and 39, respectively. Allogeneic mixed lymphocyte reactions (MLRs) were utilized to assess in vitro alloreactivity.
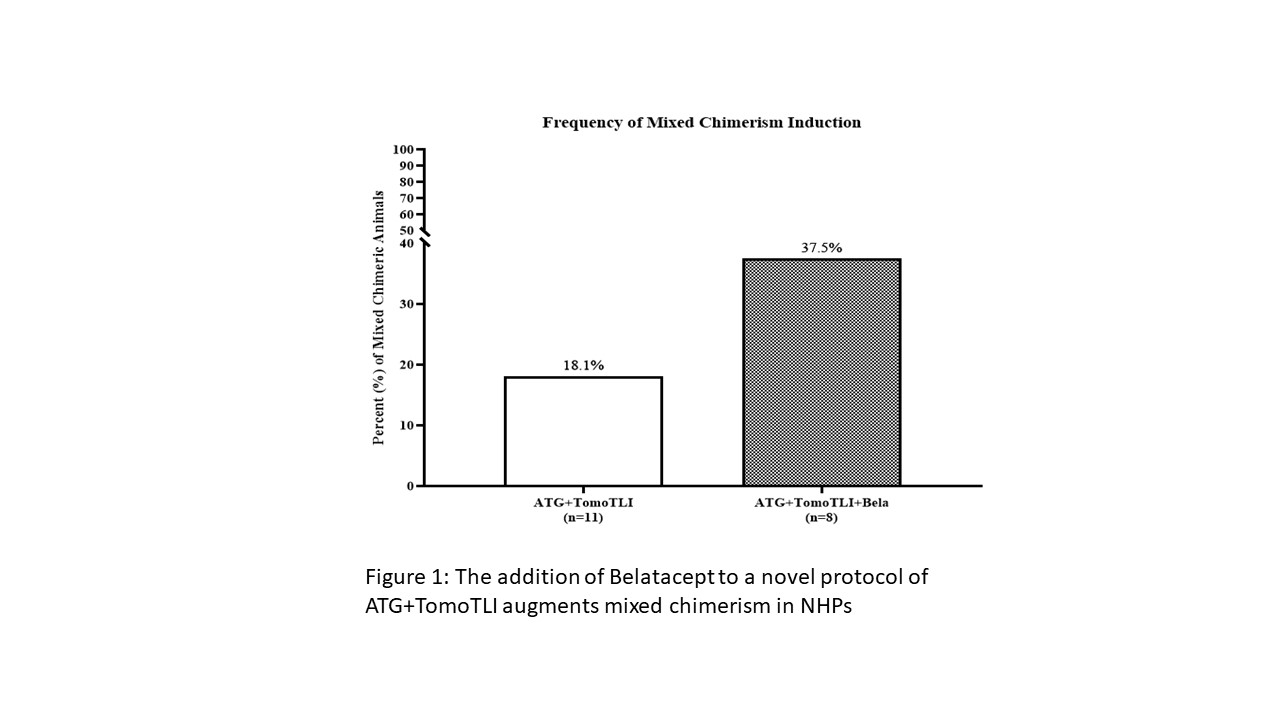
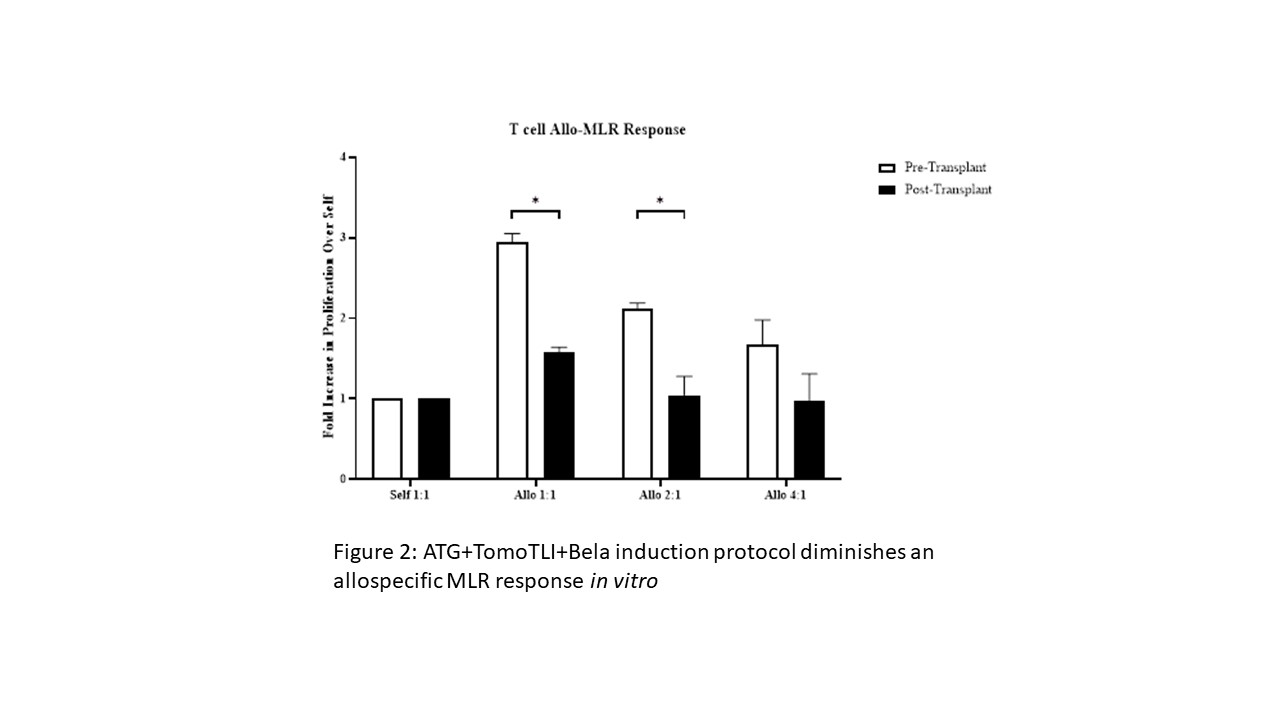
*Results: ATG+TomoTLI (n=11) is more effective at immediate and sustained lymphocyte depletion at post conditioning days 7 (p=0.003), 14 (p<0.001), and 21 (p=0.006) compared to ATG induction therapy alone (n=2); however, there was a high rate of early (30 day) DSA production and resultant non-engraftment in 9 of 11 recipients. The addition of Belatacept (n=8) eliminated early DSA production, which yielded a higher frequency of transient mixed chimerism (18.1% vs 37.5%) (Figure 1). Furthermore this induction regimen consistently abated an early allo-MLR response 30-60 days after therapy irrespective of engraftment (p=0.008) (Figure 2), similar to operationally tolerant ATG+TomoTLI animals with a negative MLR response off all maintenance immunosuppression.
*Conclusions: The addition of Belatacept to ATG+TomoTLI maintains effective lymphocyte depletion while mitigating early DSA production, thus promoting allogeneic tolerance in a novel model of mixed chimerism-based operational tolerance in NHPs undergoing MHC-mismatched kidney transplantation.
To cite this abstract in AMA style:
Kim SC, Little CJ, Fechner JH, Chlebeck PJ, Post J, D'Alessandro A, Kaufman DB. Belatacept Augments Mixed Chimerism in a Novel Nonhuman Primate Kidney Transplant Tolerance Induction Protocol [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-augments-mixed-chimerism-in-a-novel-nonhuman-primate-kidney-transplant-tolerance-induction-protocol/. Accessed July 13, 2025.« Back to 2022 American Transplant Congress