Alloreactive Regulatory T Cells Release Distinct Subpopulations of Extracellular Vesicles Bearing Immune Regulatory Proteins and the TCR/CD3 Complex Under Antigen Specific Stimulation
Brigham and Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 581
Keywords: Kidney transplantation, T cell receptors (TcR), T cells, Tolerance
Topic: Basic Science » Basic Science » 12 - Immunosuppression & Tolerance: Preclinical & Translational Studies
Session Information
Session Name: Tregs and Tolerance
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:40pm-5:50pm
Presentation Time: 5:40pm-5:50pm
Location: Hynes Room 304 / 306
*Purpose: Extracellular vesicles (EVs) are vital mediators of intercellular communication, playing an important role in the immune synapse and the T cell response. However, regulatory T cell (Treg)-EV protein cargo has not been extensively characterized. Using an in-house protocol for expanding donor alloreactive Treg-enriched cell lines (ASTRLs), we have shown that Treg EVs exert significant suppressive function. To better understand and define the underlying mechanisms of Treg EV-mediated suppression, we performed, for the first time, an in-depth proteomic analysis of human Treg-derived EVs released in response to allopeptide stimulation.
*Methods: Culture supernatant was collected from ASTRL cell lines under stimulation and rest conditions. Large and small EV fractions (lgEV and smEV) were isolated via differential ultracentrifugation and characterized by liquid chromatography-mass spectrometry. Statistical analysis/visualization was performed using R and Qlucore Omics Explorer. Gene ontology and pathway enrichment analysis were performed using Consensus Path DB and ENRICHR.
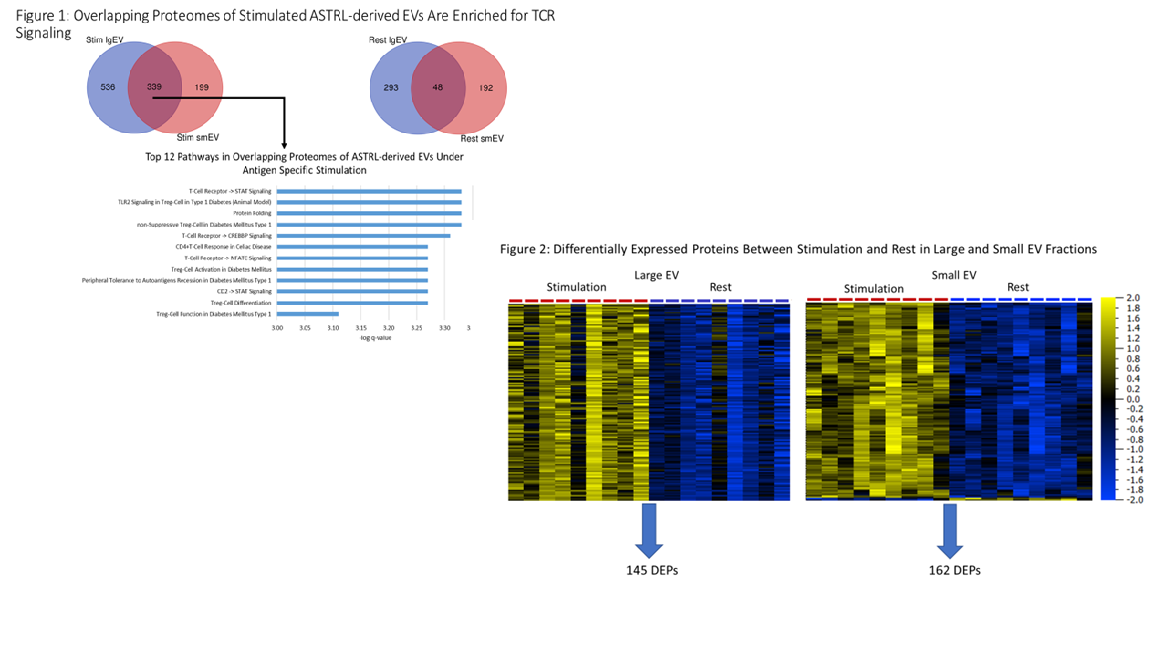
*Results: Global proteomics identified 1,709 unique proteins in the lgEV fraction and 1,356 unique proteins in the smEV fraction, 753 and 748 of which, respectively, are known to be EV-associated. Pathway analysis revealed that both lgEV and smEV fractions under stimulation were enriched for proteins involved in T cell receptor (TCR) signaling and Treg differentiation including CD3ζ and ZAP70 (Fig. 1). However, under rest conditions, only the lgEV fraction contained TCR signaling proteins. Comparison of stimulated and rest states in each size fraction identified 145 differentially enriched proteins (DEPs) in the lgEVs and 162 DEPs in the smEVs (q<0.05; Fig. 2). Pathway analysis of DEPs showed differences between lgEVs and smEVs with lgEVs containing cargo involved in oxidative phosphorylation and mitochondrial protein transport and smEVs containing immune-associated proteins involved with MHC II antigen presentation.
*Conclusions: Tregs release EVs containing activated TCR complex upon stimulation suggesting antigen specific targeting. However, differences in EV cargo imply distinct suppressive mechanisms are used by different subpopulations. These findings provide insight into Treg EV-mediated mechanisms of antigen specific immune suppression and may facilitate development of Treg EV-based therapeutics.
To cite this abstract in AMA style:
Schreiber B, Tripathi S, Sonawane A, Blaser M, Kuraoka S, Singh S, Aikawa M, Aikawa E, Chandraker A. Alloreactive Regulatory T Cells Release Distinct Subpopulations of Extracellular Vesicles Bearing Immune Regulatory Proteins and the TCR/CD3 Complex Under Antigen Specific Stimulation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/alloreactive-regulatory-t-cells-release-distinct-subpopulations-of-extracellular-vesicles-bearing-immune-regulatory-proteins-and-the-tcr-cd3-complex-under-antigen-specific-stimulation/. Accessed July 18, 2025.« Back to 2022 American Transplant Congress