A Model of Idiopathic Pneumonia Syndrome in Rhesus Macaques
1Pulmonary & Critical Care Medicine, Massachusetts General Hospital, Boston, MA, 2Hematology/Oncology, Boston Children's Hospital, Boston, MA, 3Pathology, Massachusetts General Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1516
Keywords: Graft-versus-host-disease, Histology, Lung, Primates
Topic: Basic Science » Basic Science » 05 - Translational Cellular Therapies: Islet and Stem Cell Transplantation
Session Information
Session Name: Translational Cellular Therapies: Islet and Stem Cell Transplantation
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: To create a model of idiopathic pneumonia syndrome (IPS), a deadly complication of hematopoietic stem cell transplantation (HSCT) in Rhesus macaques (RM).
*Methods: RM were conditioned with 1200 cGy total body irradiation and received either allogeneic, MHC-mismatched or autologous T-cell replete G-CSF-mobilized leukapheresis products without anti-Graft-versus-Host-Disease prophylaxis. Lungs were retrieved from recipients at day +8 post-HSCT or from healthy non-transplant control animals and scored by quantifying the number of blood vessels with perivascular infiltration (vascular inflammation index; VII) or with mononuclear infiltration protruding into the interstitium (interstitial pneumonitis index; IPI). Transcriptomic analysis of CD3+CD20- lung T cells was performed using RM-specific whole genome microarrays, followed by differentially-expressed gene (DEG) identification and pathway enrichment analysis.
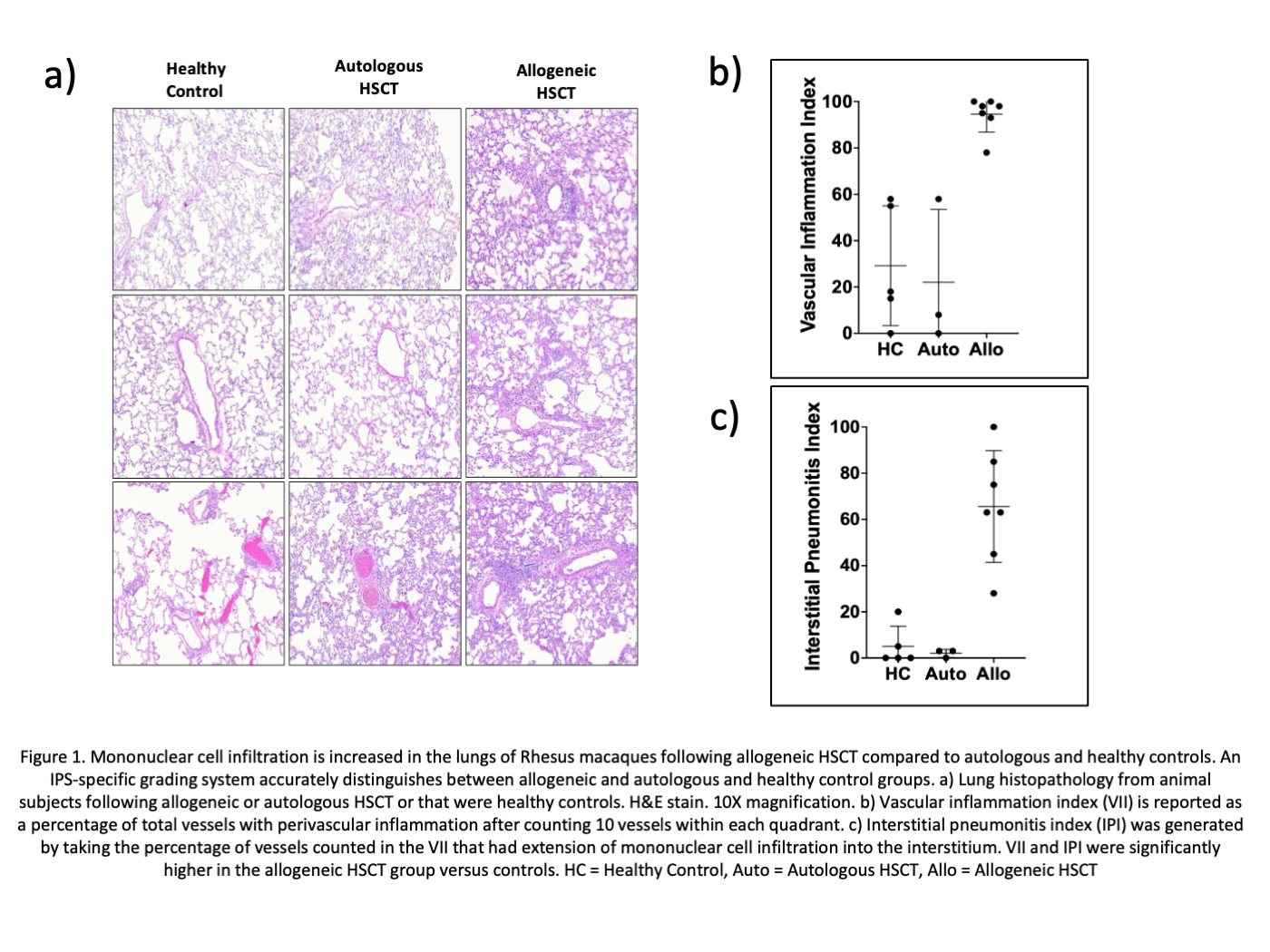
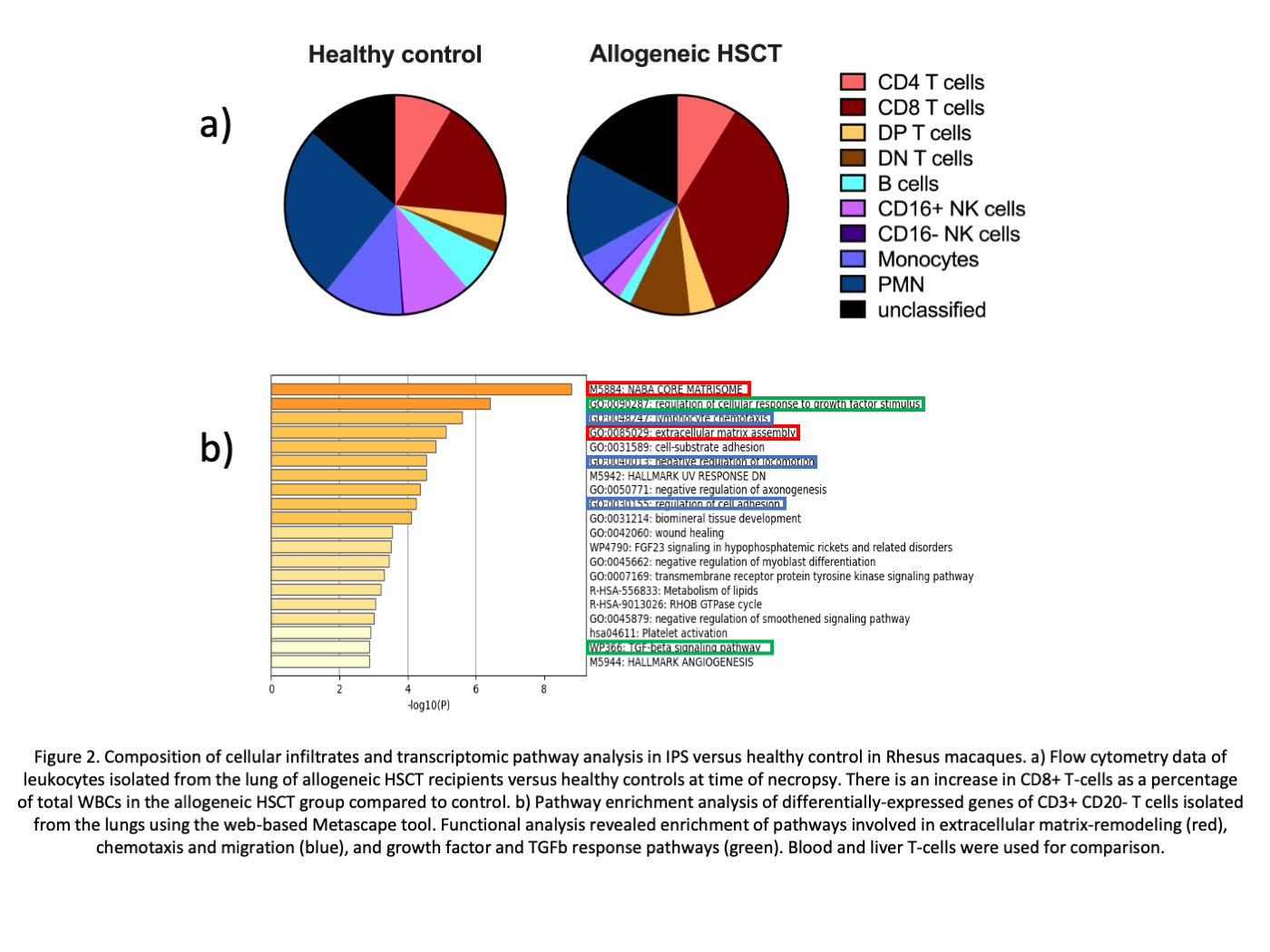
*Results: Histopathological analysis revealed marked increases in mononuclear cell infiltration in the lungs of allogeneic HSCT subjects compared to autologous HSCT recipients or healthy controls (Figure 1a) with significantly higher VII (94.6 vs 22.0 vs 29.2, p<0.001; Figure 1b) and IPI (65.6 vs 2.0 vs 5.0, p<0.001; Figure 1c). Mononuclear cell infiltrates in the lungs after allogeneic HSCT were enriched for CD8+ T cells (Figure 2a). We identified 187 DEG that were specific for lung allogeneic HSCT cohort. Functional analysis of these DEGs revealed enrichment for extracellular matrix-remodeling, chemotaxis/migration, and growth factor (including TGFb) response pathways (Figure 2b).
*Conclusions: Allogeneic HSCT recipients demonstrated higher degrees of pulmonary inflammation compared to autologous HSCT and healthy control animals, which was driven by CD8+T cells that are characterized by a tissue-specific transcriptomic signature. We have established a reliable model of IPS in RM that allows thorough mechanistic studies to discover underlying immunological mechanisms.
To cite this abstract in AMA style:
Bermea RS, Ingersoll K, Hariri LP, Warren M, Kean L, Tkachev V. A Model of Idiopathic Pneumonia Syndrome in Rhesus Macaques [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/a-model-of-idiopathic-pneumonia-syndrome-in-rhesus-macaques/. Accessed July 15, 2025.« Back to 2022 American Transplant Congress