Utilizing Ides To Eliminate Residual Antibodies Targeting Xenografts
Massachusetts General Hospital, Boston, MA
Meeting: 2019 American Transplant Congress
Abstract number: 273
Keywords: Antibodies, Immunogenicity, Pig, Xenoreactive antibodies
Session Information
Session Name: Concurrent Session: Xenotransplantation
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 310
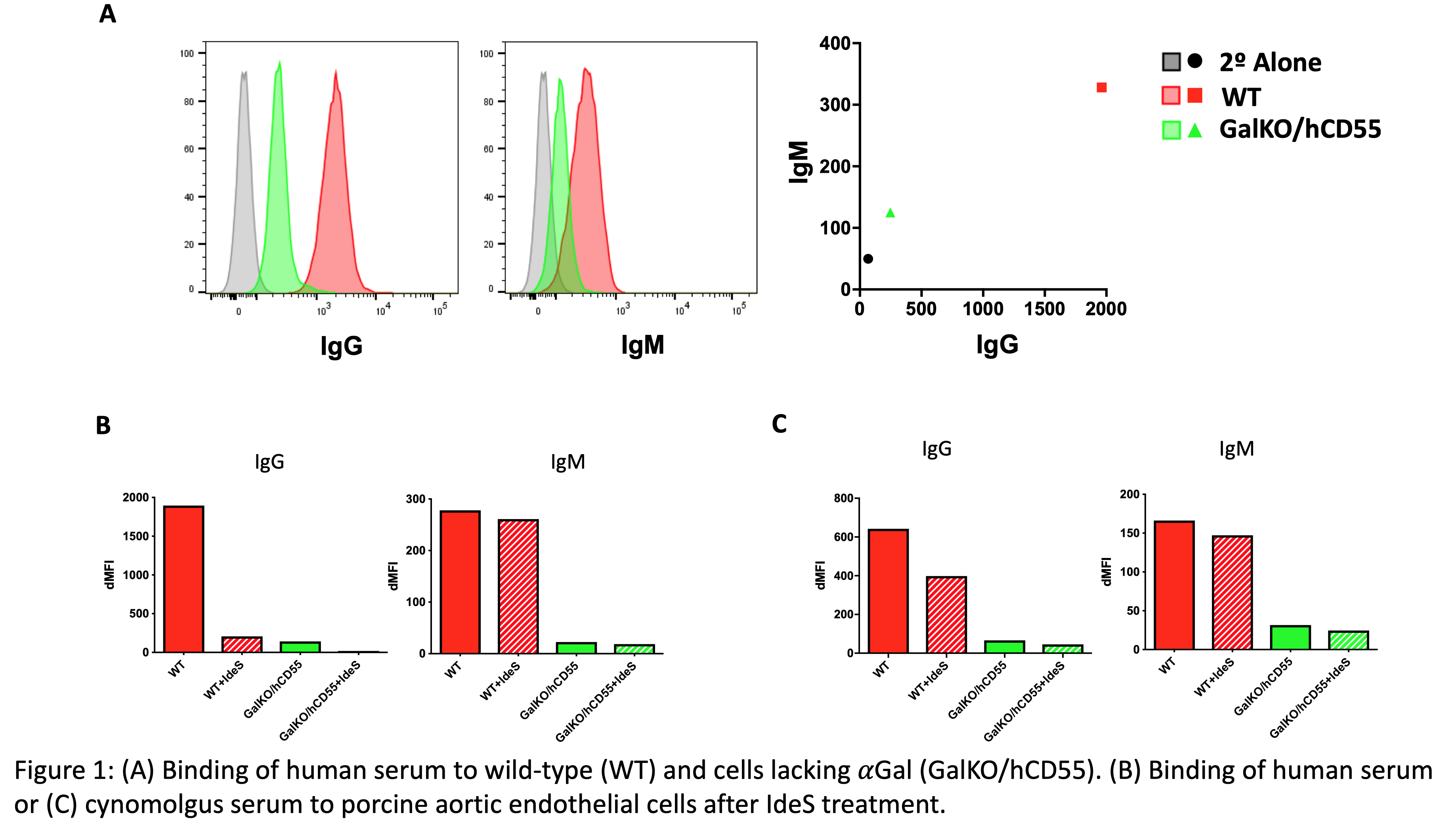
*Purpose: A significant hinderance to the successful use of xenotransplantation has been the presence of preformed antibodies against the donor antigens, known as xenoantigens. Using modern genetic engineering tools, researchers have been able to eliminate expression of the most commonly identified xenoantigens from pig strains utilized for organ transplantation. However, experiments show that antibody binding to these genetically modified organs persists at a low level. Here, we perform a proof-of-concept experiment to demonstrate that the antibody cleaving protease, IdeS, is capable of eliminating residual IgG binding to genetically modified porcine cells.
*Methods: Human or cynomolgus macaque serum was treated with the IdeS enzyme (FABricator, Genovis Inc.) for 30 minutes per manufacture guidelines to cleave IgG into separate Fc and F(ab’)2 fragments. Treated and untreated serum were utilized to stain cultured primary porcine aortic endothelial cells (pAEC) from WT pigs or genetically modified pigs, lacking the xenoantigen, αGal. Using flow cytometry, fluorophor-conjugated secondary antibodies against either IgG or IgM were utilized to assess the degree of intact antibody binding to the pAEC.
*Results: Genetically modified pAEC, lacking αGal, showed a roughly 90% reduction of both IgG and IgM binding from human serum compared to WT pAEC (figure 1A). IdeS treatment further reduces the level of IgG binding to modified pAEC to background levels for human serum and to a similar but lesser extent for monkey serum (figure 1B,C). As expected, because IdeS is an IgG-specific protease, the treatment had no effect on the level of IgM binding.
*Conclusions: IdeS treatment of serum is capable of eliminating residual binding of functional IgG to genetically modified porcine cells, suggesting that it is a potential tool to decrease antibody binding to xenotransplant organs and eliminate antibody- and complement-mediated graft injury.
To cite this abstract in AMA style:
Rickert C, Lee K, Tanimine N, Lei J, Cuenca A, Feeney N, Deng K, Yeh H, Markmann JF. Utilizing Ides To Eliminate Residual Antibodies Targeting Xenografts [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/utilizing-ides-to-eliminate-residual-antibodies-targeting-xenografts/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress