To Each Their Own: Center-Level Variation in Tailoring Induction Immunosuppression to Kidney Transplant Recipients
Johns Hopkins University, Baltimore, MD
Meeting: 2019 American Transplant Congress
Abstract number: 559
Keywords: Antilymphocyte antibodies, Induction therapy, Interleukin-2 receptor, Multicenter studies
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Induction Therapy
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 304
*Purpose: Ideally, induction immunosuppression should be selected in a personalized manner. However, there is little knowledge of what clinical factors are considered in this process across the country. An understanding of how centers vary in determining induction regimen is crucial to inform research on optimal personalization. We aimed to quantify the center-level variation in how each clinical factor affects induction selection.
*Methods: Using SRTR data, we studied 139,673 kidney transplant recipients in 2002-2017 who received anti-thymocyte globulin (ATG) or interleukin-2 receptor antagonist (IL2RA) for induction and tacrolimus and mycophenolate for maintenance immunosuppression. Using multi-level logistic models, we estimated the odds of receiving ATG by 65 variables representing 32 characteristics. Predictor variables were normalized to have means of 0 and standard deviations (SD) of 1.
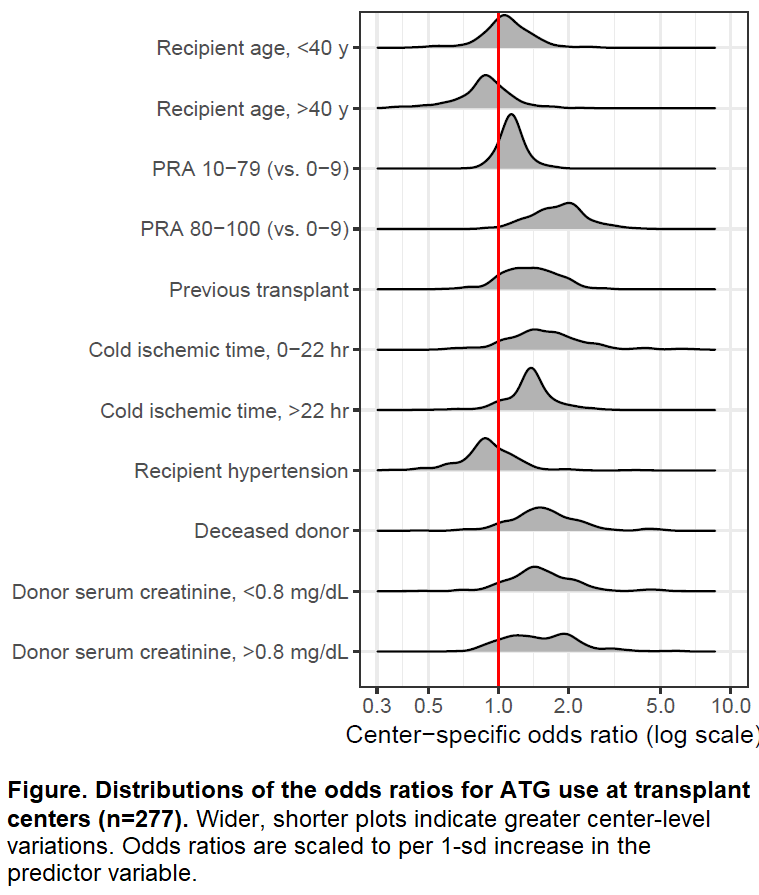
*Results: Of 32 characteristics, 7 (donor serum creatinine, cold ischemic time, deceased vs living donor, recipient age, recipient hypertension, PRA, and recipient history of previous transplants) had variance exceeding 0.34, indicating the greatest disagreement between centers on how these variables should be considered in induction tailoring [Table]. For instance, across the entire population, older recipient age was associated with lower odds of ATG use [OR=0.810.860.91; per 10.9 y (1 SD) increase after 40 y]. However, when estimated specifically for each center, this odds ratio was greater than 1.0 in 72 (26.0%) centers, indicating older recipient age was associated with greater odds of ATG use in these centers [Figure].
*Conclusions: There is a substantial center-level variation in personalizing induction immunosuppression, and practices at different centers are contradictory. An evidence-based guidance on this practice is warranted.
| Recipient | age, race (White, Black, and others), sex, PRA, previous transplants, BMI, diabetes, hypertension, cause of ESRD, preemptive transplants, time on dialysis, serum albumin, PVD, malignancy, HCV, insurance. |
| Donor | age, race (White, Black, and others), sex, type (deceased vs living), BMI, HCV. For deceased donors – donation after cardiac death, diabetes, hypertension, cause of death, machine perfusion, serum creatinine. |
| Transplant | HLA-A/B/DR mismatches, cold ischemic time. |
To cite this abstract in AMA style:
Bae S, Massie A, Wang JGaronzik, DeMarco MMcAdams, Coresh J, Segev D. To Each Their Own: Center-Level Variation in Tailoring Induction Immunosuppression to Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/to-each-their-own-center-level-variation-in-tailoring-induction-immunosuppression-to-kidney-transplant-recipients/. Accessed February 23, 2026.« Back to 2019 American Transplant Congress