Systematic Evaluation of Immunosuppressant Drug Tolerability in Lung Transplant Recipients with Short Telomere Syndrome
1Department of Pharmacy, University of Chicago Medicine, Chicago, IL, 2Department of Medicine, Division of Pulmonology, University of Chicago Medicine, Chicago, IL
Meeting: 2021 American Transplant Congress
Abstract number: 817
Keywords: Adverse effects, Calcineurin, Neutropenia, Thrombocytopenia
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
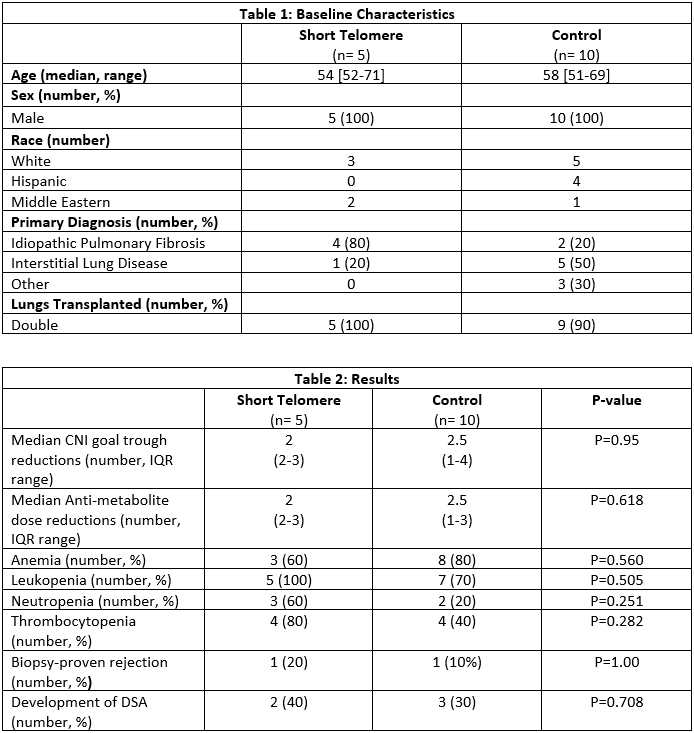
*Purpose: Lung transplant recipients with short telomere syndrome are at high risk of hematologic complications. Consequently, patients with short telomere syndrome may require more frequent dose adjustments and an overall reduction of immunosuppression after transplant. It is not known if this is linked with an immunologic consequence such as higher rejection rates or development of donor specific antibody (DSA).
*Methods: This was a single center, retrospective, 2:1 matched cohort study. Patients were matched for transplant date within 1 year, age at transplant within 5 years, and sex. Patients who survived less than 1 year post-transplant were excluded. Data were collected for 1 year post-transplant on all patients, with the exception of one short telomere patient and two matched controls who had not yet reached the one-year mark. Primary outcomes were median number of calcineurin inhibitor (CNI) target trough reductions and anti-metabolite dose reductions. Additional outcomes included number of patients experiencing severe anemia (hemoglobin < 8 grams per deciliter), leukopenia (white blood cell count < 3000 cells per cubic millimeter), neutropenia (absolute neutrophil count < 1000 cells per microliter), thrombocytopenia (platelet count < 100,000 per microliter), biopsy proven rejection (BPAR), and development of DSA.
*Results: Fifteen patients were included in the study, 5 patients with short telomere syndrome and 10 matched controls. Baseline characteristics are summarized in Table 1 and study outcomes in Table 2. There was no difference in the median number of CNI target trough reductions (2 vs. 2.5, p=0.95) or median number of anti-metabolite dose reductions (2 vs. 2.5, p=0.618). While a greater percentage of short telomere patients experienced leukopenia, neutropenia, and thrombocytopenia, it did not reach statistical significance in this small sample. There was no difference in BPAR or DSA.
*Conclusions: Short telomere patients did not require significantly more dose adjustments to immunosuppressant medications or experience significantly more adverse effects when compared to non-short telomere patients 1-year post-transplant.
To cite this abstract in AMA style:
Patolia R, Guzy R, Potter L. Systematic Evaluation of Immunosuppressant Drug Tolerability in Lung Transplant Recipients with Short Telomere Syndrome [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/systematic-evaluation-of-immunosuppressant-drug-tolerability-in-lung-transplant-recipients-with-short-telomere-syndrome/. Accessed March 6, 2026.« Back to 2021 American Transplant Congress