Safety and Efficacy of Prophylactic Valganciclovir Dosing after Solid Organ Transplant
K. Gandhi, D. Masic, M. Santarossa, F. Albarillo, N. Clark, G. Reid
Loyola University Medical Center, Maywood, IL
Meeting: 2020 American Transplant Congress
Abstract number: A-188
Keywords: Cytomeglovirus, Heart/lung transplantation, Lung transplantation, Neutropenia
Session Information
Session Name: Poster Session A: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: At centers where antiviral prophylaxis is used after solid organ transplant, valganciclovir is administered for the prevention of cytomegalovirus (CMV). At Loyola University Medical Center (LUMC), most thoracic transplant recipients receive valganciclovir 450 mg twice daily (BID), while most abdominal transplant recipients receive 900 mg daily (QD) for CMV prophylaxis in patients with creatinine clearance (CrCl) greater than 60 mL/min. The purpose of this study is to analyze the safety and efficacy of two different prophylactic valganciclovir dosing strategies after solid organ transplant.
*Methods: This was a retrospective study of heart, lung, and liver transplant recipients who received valganciclovir for CMV prophylaxis from August 2014 to August 2019. Patients were excluded if CrCl was less than 60 mL/min. The primary endpoint was incidence of neutropenia. The secondary endpoint was incidence of CMV infection, which was defined as having a detectable serum CMV viral load. Patient follow-up occurred within the study period.
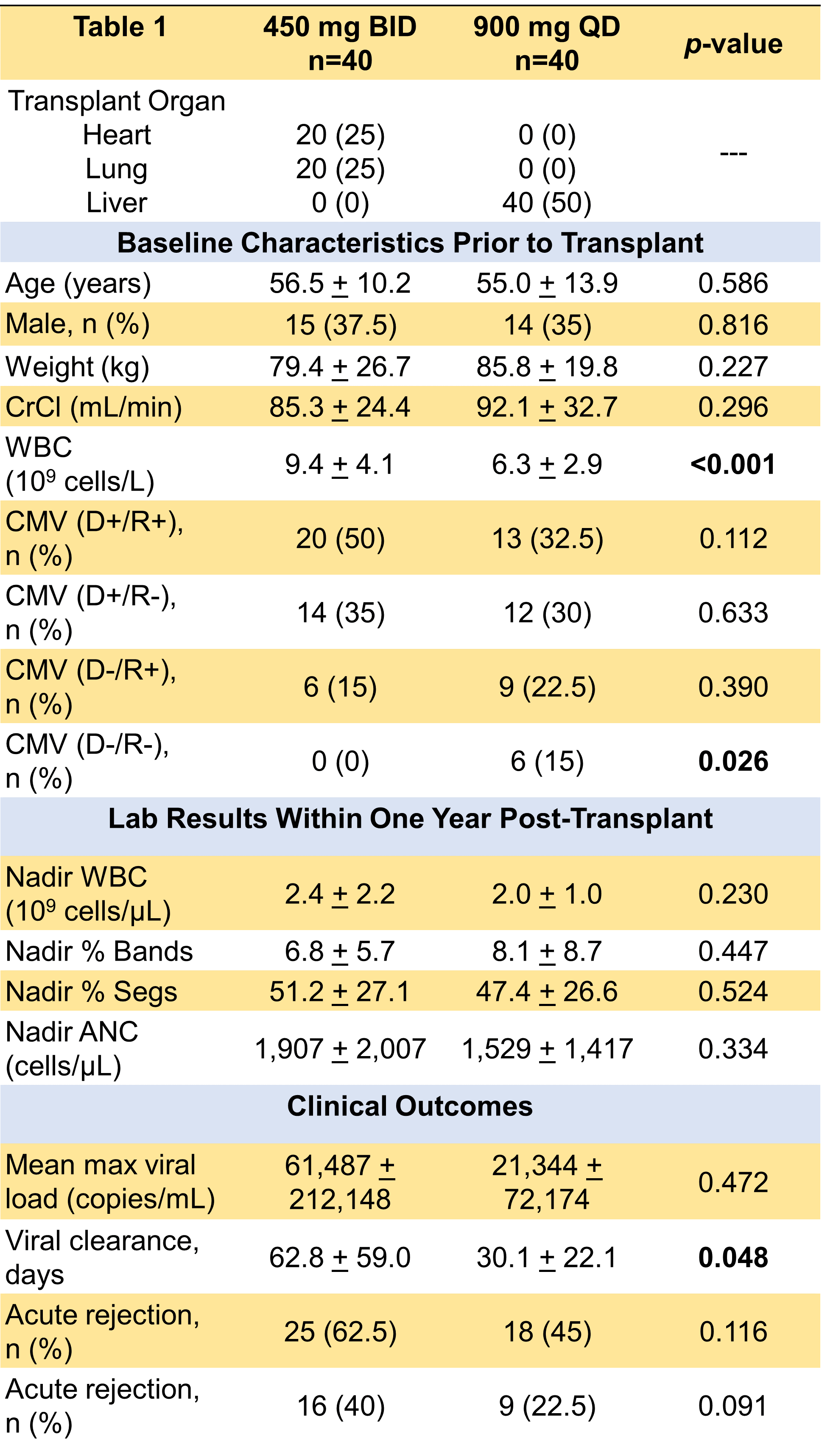
*Results: Eighty patients were included in the analysis, with 40 patients receiving valganciclovir 900 mg QD and 40 patients receiving 450 mg BID. Baseline characteristics were similar, except there was a significantly lower baseline WBC in the 900 mg QD group (Table 1). There were no significant differences in post-transplant lab results or incidence of acute rejection (suspected and/or confirmed). The incidence of CMV infection was more prevalent in the 450 mg BID group, and the 450 BID group also had a longer time to undetectable CMV viral load (Table 1). There was no significant difference in the incidence or severity of neutropenia post-transplant between both prophylactic dosing strategies.
*Conclusions: Both 450 mg BID and 900 mg QD dosing strategies of prophylactic valganciclovir appear to be safe and effective. Differences in incidence of CMV infection and time to viral clearance were found between the two groups in this study. However, a major limitation is that the majority of patients in 450 mg BID group were thoracic transplant recipients and majority of patients in the 900 mg QD group were liver transplant recipients. Since inherent differences exist between these two groups with regards to incidence and severity of CMV infection and disease, more research is warranted.
To cite this abstract in AMA style:
Gandhi K, Masic D, Santarossa M, Albarillo F, Clark N, Reid G. Safety and Efficacy of Prophylactic Valganciclovir Dosing after Solid Organ Transplant [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-prophylactic-valganciclovir-dosing-after-solid-organ-transplant/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress