IL-10 Expression of In Vitro Activated B Cells as A Biomarker for B Cell Tolerance.
Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA.
Meeting: 2016 American Transplant Congress
Abstract number: D89
Keywords: B cells, Mixed chimerism, T cells, Tolerance
Session Information
Session Name: Poster Session D: Clinical Science: Tolerance: Clinical Studies
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background: A significant role for regulatory B cell has been suggested in kidney transplant recipients who achieved operational tolerance while receiving conventional immunosuppression. We studied T and B cell responses in recipients in whom tolerance induction was attempted via HLA-mismatched combined kidney and bone marrow transplantation (CKBMT).
Material/Methods: Five CKBMT recipients who achieved long-term immunosuppression-free survival (>5 years) were retrospectively studied. Two recent CKBMT recipients were also prospectively studied. T-cell responses were determined by IFN-g ELIspot assay using irradiated PBMCs (direct) or lysates (indirect). B cell responses were evaluated by IL-10 expression of naïve B-cells after stimulation with CD40L/IL-2/CpG/F(ab)'.
Results: Donor specific antibodies (DSA) were never detected in four recipients but three developed low (n=2) or high titer (n=1) DSA. Nevertheless, except for the recipient with high DSA titers, all recipients showed donor-specific hyporesponsiveness in direct IFN-g ELIspot (Fig. 1A). No anti-donor responses were detected by indirect IFN-g ELIspot. In contrast, significantly higher IL-10 expression was observed on in vitro activated B cells obtained from long-term recipients with no DSA than from recipients with DSA (Fig. 1B). Similar observations were made in the two prospectively followed recipients, one of whom achieved tolerance without DSA while the other developed subclinical rejection and low titer DSA, first detected on day 518, during weaning of his immunosuppression. Although both recipients showed donor-specific hyporesponsiveness on direct IFN-g ELIspot assay, IL-10 expression of in vitro activated B cells was noted to fall in the rejecting recipient. In contrast, IL-10 expression remained high in recipient who achieved tolerance (Fig. 1C).
Conclusion: Although T cell response assay failed to differentiate “Tolerant” from “non-Tolerant” recipients, IL-10 expression of in vitro activated B cells appear to be a useful biomarker for B cell tolerance. Studies to identify the B cell specific subsets responsible for the higher IL-10 expression are currently underway.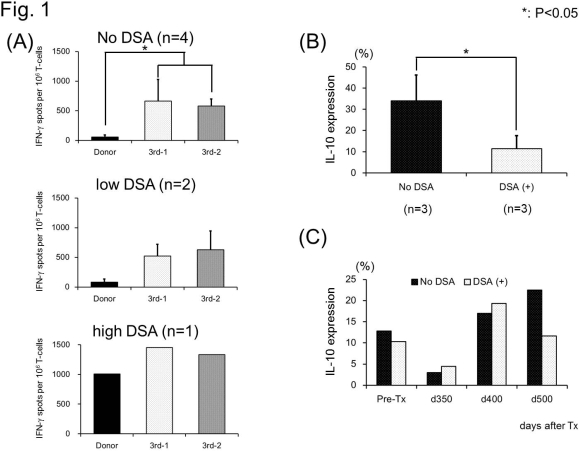
CITATION INFORMATION: Oura T, Hotta K, Crisalli K, Cosimi B, Kawai T. IL-10 Expression of In Vitro Activated B Cells as A Biomarker for B Cell Tolerance. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Oura T, Hotta K, Crisalli K, Cosimi B, Kawai T. IL-10 Expression of In Vitro Activated B Cells as A Biomarker for B Cell Tolerance. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/il-10-expression-of-in-vitro-activated-b-cells-as-a-biomarker-for-b-cell-tolerance/. Accessed February 24, 2026.« Back to 2016 American Transplant Congress
