Ex Vivo Expansion and Functional Characterization of Human Alloantigen-Specific Regulatory T Cells.
1Surgery - Comprehensive Transplant Center, Northwestern University, Chicago, IL
2Multimeric Biotherapeutics, Inc, La Jolla, CA.
Meeting: 2016 American Transplant Congress
Abstract number: D87
Keywords: Alloantigens, Preclinical trails, T cells, Tolerance
Session Information
Session Name: Poster Session D: Clinical Science: Tolerance: Clinical Studies
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Purpose: Regulatory T cells (Tregs) have been associated with clinical transplant tolerance and they have been shown to prolong graft survival in animal models without immunosuppression. Therefore, a number of centers have initiated clinical trials with ex vivo expanded Tregs. The purpose of the present study was to expand Tregs using activated/expanded allogeneic B cells and test their antigen (Ag)-specific function.
Methods & Results: Peripheral blood mononuclear cells (PBMC) from healthy volunteers were cultured using novel stimulating agent multimeric Ultra-CD40L, under B cell-promoting conditions in presence of cyclosporine A to prevent T cell growth (n=8). The resultant B cells expanded 100-1,000 fold by day 14-21 and displayed increased expression of CD80, CD86, and HLA-DR, the hallmark of mature antigen presenting cells. These expanded B cells, after irradiation were used to stimulate purified CD4+CD127–CD25+ Tregs isolated from PBMC of an allogeneic volunteer for 28 days under Treg-promoting conditions with exogenous IL-2 and sirolimus (SRL). The Tregs expanded rapidly when SRL was removed (Fig. A). They maintained the CD4+CD127–CD25+ phenotype with >60% being FOXP3+. More importantly, they dose-dependently inhibited MLR of autologous responders in an ag-specific manner (Fig. B). Additionally, statistically significant inhibition occurred even at 1:250 with Ag-specific Tregs vs only up to 1: 32 when non-specifically expanded polyclonal Tregs were used (0% inhibition controls being autologous PBMC). The Ag-specific Tregs also demonstrated infectious tolerance by generating new Tregs in CFSE-labeled autologous responder cells cultured with the specific allostimulator (n=6). 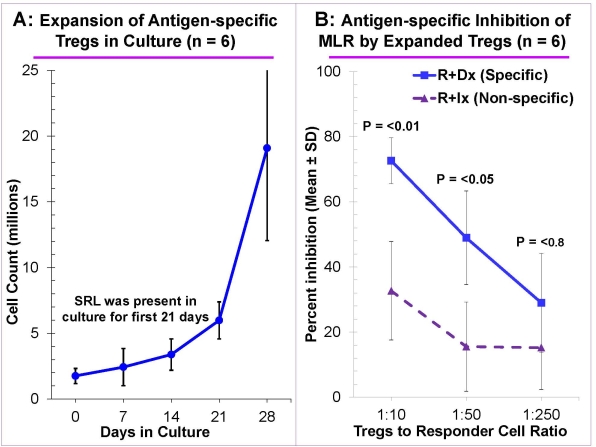
Conclusions: Ultra-CD40L activated/expanded B cells can be utilized to grow alloantigen-specific Tregs which are powerful Ag-specific immunomodulators of autologous responding cells. Upon further development and scale-up under GMP conditions, such Tregs could be more effectively utilized to achieve clinical transplant tolerance.
CITATION INFORMATION: Mathew J, McEwen S, Konieczna I, Huang X, He J, Kornbluth R, Miller J, Gallon L, Leventhal J. Ex Vivo Expansion and Functional Characterization of Human Alloantigen-Specific Regulatory T Cells. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Mathew J, McEwen S, Konieczna I, Huang X, He J, Kornbluth R, Miller J, Gallon L, Leventhal J. Ex Vivo Expansion and Functional Characterization of Human Alloantigen-Specific Regulatory T Cells. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/ex-vivo-expansion-and-functional-characterization-of-human-alloantigen-specific-regulatory-t-cells/. Accessed March 7, 2026.« Back to 2016 American Transplant Congress
