Enumeration of Platelets by Flow Cytometry Reveals Human Erythrocyte Fragmentation During Pig Organ Xenoperfusion Studies
1Surgery, Massachusetts General Hospital and Harvard School of Medicine, Boston, MA, 2Surgery, University of Maryland School of Medicine, Baltimore, MD, 3eGenesis, Cambridge, MA
Meeting: 2020 American Transplant Congress
Abstract number: A-269
Keywords: FACS analysis, Methodology, Pig, Thrombocytopenia
Session Information
Session Name: Poster Session A: Xenotransplantation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Human or baboon platelet sequestration and activation occurs during pig organ xenograft perfusion, although the mechanism remains poorly understood. To evaluate an apparent “rebound” of platelet counts that was repeatedly observed in multiple preclinical experimental xenotransplantation models, we developed a flow cytometry-based technique to accurately quantify platelets.
*Methods: Heparinized human whole blood was perfused through seven pairs (n=14) of lungs from transgenic pigs with Gal1,3αGal, β4Gal, and Neu5Gc knockouts and containing human transgenes addressing molecular incompatibilities in complement activation, and innate and adaptive immune cell function (Pig 2.0). Pigs were pretreated with DDAVP, and blood perfusate was left untreated (n=7) or treated with a thromboxane synthase inhibitor and histamine receptor blocker (n=7). Platelets were enumerated using an Heska (Hematru) traditional hemocytometer (HC), and a refined flow cytometric method (FC) using calibrated beads. FC results analyzed by FlowJo were compared with HC results.
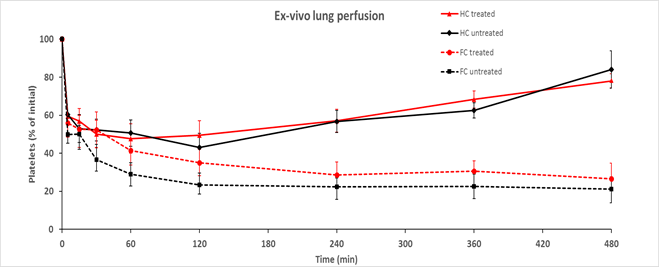
*Results: With the HC method, platelet counts rapidly decreased after perfusion and remained low for at least 2hr, an apparent “rebound” typically observed after 240 minutes (Figure 1, solid lines). In contrast, platelet counts by FC showed a continuing decrease throughout each experiment (dotted lines). FC analysis revealed an increasing population of platelet-sized FC events which were negative for human platelet markers (CD41, CD61). Using antibodies for additional human leukocyte (CD45), erythrocyte (CD235a), and pig-specific antibodies (CD41, CD45, CD31), a majority of platelet-sized CD41-negative events express the CD235a red blood cell marker.
*Conclusions: FC offers improved accuracy of platelet enumeration relative to conventional HC and reveals that overestimation of platelet counts by HC results primarily from erythrocyte fragmentation, with a minor contribution from other cellular debris. The mechanism driving human erythrocyte fragmentation during pig organ perfusion remains under active study. We speculate that red blood cell injury may contribute to the anemia which is frequently observed in in vivo pig-to-primate organ xenograft recipients.
To cite this abstract in AMA style:
Abady Z, Petitpas KM, Burdorf L, Pratts SG, Cerel BM, Sendil S, Connolly MR, Qin W, Kan Y, Layer J, Youd M, Westlin W, Yang L, III RNPierson, Azimzadeh AM. Enumeration of Platelets by Flow Cytometry Reveals Human Erythrocyte Fragmentation During Pig Organ Xenoperfusion Studies [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/enumeration-of-platelets-by-flow-cytometry-reveals-human-erythrocyte-fragmentation-during-pig-organ-xenoperfusion-studies/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress