Enhanced IL-6 Production Impedes Transplant Tolerance Induction in Lupus-Prone Mice.
Pediatrics and Pathology, Microbiology, and Immunology, Vanderbilt University, Nashville, TN.
Meeting: 2016 American Transplant Congress
Abstract number: 294
Keywords: Gene polymorphism, Islets, Mice, Tolerance
Session Information
Session Name: Concurrent Session: Challenges to Graft Survival and Tolerance: Animal Models
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: Room 306
Significance: Autoimmunity is a significant barrier to transplantation. Based on previous studies, it is apparent that there are separate genetic regions that control susceptibility to autoimmunity vs resistance to transplantation tolerance, but these have been difficult to isolate in polygenic models. We have recently established that B6.SLE123 mice, in which a lupus phenotype is conferred by 3 genetic regions, resist tolerance induction to islet transplants in absence of any pre-existing autoimmunity. Using this congenic model, we now further trace which genetic regions contribute to tolerance resistance and their mechanisms.
Methods: Single congenic B6.SLE1, B6.SLE2, and B6.SLE3 mice were made diabetic, transplanted with C3H islets, and treated with anti-CD45RB (100ug/day, days 0,1,3,5,7). Rejection was detemrined by two consecutive BG values > 250mg/dL. Evaluation of the alloresponse was measured via ex vivo and in vivo mixed lymphocyte reactions (MLRs). IL-6 production was assessed via ELISA. IL-6 signaling blockade was achieved by administering 500ug of anti-IL6R on days -3,-1,1,3 relative to transplant; blockade efficacy was evaluated via phosphoflow cytometry.
Results: Each single congenic strain resisted anti-CD45RB mediated transplant tolerance induction. Rejection kinetics of B6.SLE1 mice most closely resembled that of triple-congenic B6.SLE123 mice in which in no recipients achieved long-term tolerance. Lymphocytes from B6.SLE123 and B6.SLE1 mice demonstrated enhanced alloreactivity during an ex vivo MLR and enhanced IL-6 production. In vivo administration of an anti-IL-6R blocking antibody reduced IL-6 mediated phosphorylation of STAT3(Y705) and enhanced CD4 Treg expansion. Co-administration of anti-IL-6R with anti-CD45RB restored long-term tolerance induction to islet allografts in 40% of B6.SLE1 transplant recipients. 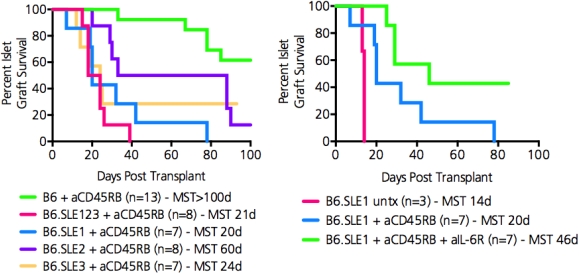
Conclusions: Analysis of minimal genetic regions from an autoimmune background demonstrate strong effects preventing transplantation tolerance. We relate the function of one of these regions in lupus to increased IL-6. IL-6 blockade may enhance organ engraftment in autoimmunity and lupus.
CITATION INFORMATION: Stocks B, Wilson C, Seto M, Marshall A, Moore D. Enhanced IL-6 Production Impedes Transplant Tolerance Induction in Lupus-Prone Mice. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Stocks B, Wilson C, Seto M, Marshall A, Moore D. Enhanced IL-6 Production Impedes Transplant Tolerance Induction in Lupus-Prone Mice. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/enhanced-il-6-production-impedes-transplant-tolerance-induction-in-lupus-prone-mice/. Accessed February 24, 2026.« Back to 2016 American Transplant Congress
