DSA After Transplant Increases Risk of Renal Allograft Failure Independent of Concurrent Biopsy Results: The DEKAF Study Data.
1UCLA Immunogenetics Center Department of Pathology, University of California-Los Angeles, Los Angeles, CA
2Department of Lab Medicine & Pathology, Mayo Clinic College of Medicine, Rochester, MN
3Department of Biostatistiscs, University of Minnesota School of Public Health, Minneapolis, MN.
Meeting: 2016 American Transplant Congress
Abstract number: 407
Keywords: Biopsy, Graft survival, HLA antibodies, Rejection
Session Information
Session Name: Concurrent Session: Kidney AMR: Predicting the Patient at Risk
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Ballroom B
Donor-specific HLA antibodies (DSA) are a major cause of renal allograft dysfunction and loss following transplant. The presence of DSA, with or without C4d deposition, together with evidence of microvascular inflammation (MVI) is required to diagnose antibody-mediated rejection using Banff criteria. We studied 294 patients transplanted at the 7 centers participating in the Deterioration of Kidney Allograft Function (DeKAF) Study with a biopsy for cause (25% increase in serum creatinine or new onset proteinuria) more than 3 months after transplant. Our objective was to determine the relative impacts of MVI and DSA at the time of biopsy on subsequent death-censored graft survival.
Biopsies were scored centrally using extended Banff criteria without clinical or DSA information. Serum samples acquired at the time of biopsy were tested at a central laboratory to identify DSA given only the donor and recipient HLA types and using single HLA antigen bead tests. Patients whose biopsy showed recurrent disease, or BK virus nephropathy as potential causes of dysfunction were excluded. Patients were followed after biopsy for an average of 3.6 years.
Death-censored graft survival (DCGS) after biopsy is illustrated in the Figure. 5-year DCGS following the biopsy was 88% among the 141 patients whose biopsy showed no evidence of MVI (ptc score=0, g score=0) and no DSA. Biopsies with evidence of MVI and no DSA (n=50), DSA with no MVI (n=39) or DSA+MVI (n=64) had significantly poorer DCGS ranging from 47-66% at 5 years (p<0.01). More than one-third of DSA+ patients in this sample had no evidence of MVI in their biopsy and 44% of patients with MVI had no detectable DSA.
We conclude that DSA testing provides important information in addition to biopsy results. Both biopsy and DSA testing should be performed to properly evaluate late renal allograft dysfunction. 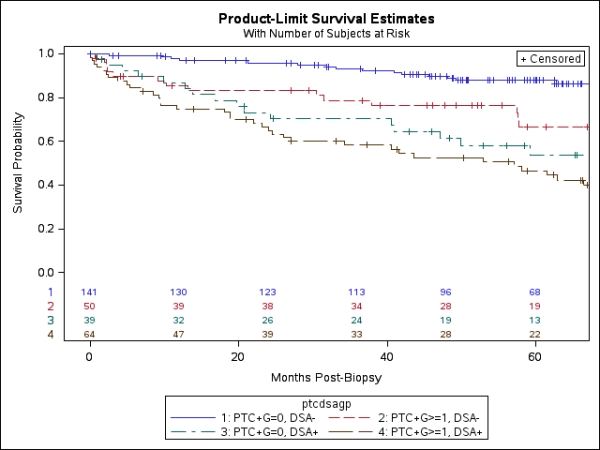
CITATION INFORMATION: Cecka J, Grande J, Cosio F, Leduc R, The DeKAF Study Group DSA After Transplant Increases Risk of Renal Allograft Failure Independent of Concurrent Biopsy Results: The DEKAF Study Data. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Cecka J, Grande J, Cosio F, Leduc R, Group TheDeKAFStudy. DSA After Transplant Increases Risk of Renal Allograft Failure Independent of Concurrent Biopsy Results: The DEKAF Study Data. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/dsa-after-transplant-increases-risk-of-renal-allograft-failure-independent-of-concurrent-biopsy-results-the-dekaf-study-data/. Accessed July 18, 2025.« Back to 2016 American Transplant Congress
