Distinctive Roles of mTORC1 Vs. mTORC2 in Liver Ischemia-Reperfusion Injury
1The Dumont-UCLA Transplant Center, UCLA, Los Angeles
2Liver Transplant Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
3Liver Surgery, Renji Hospital, Shanghai Jiaotong University, Shanghai, China.
Meeting: 2015 American Transplant Congress
Abstract number: 505
Keywords: Hepatocytes, Inflammation, Ischemia, Liver
Session Information
Session Name: Concurrent Session: Strategies To Minimize Ischemia Reperfusion Injury
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 4:24pm-4:36pm
Presentation Time: 4:24pm-4:36pm
Location: Room 119-B
Mammalian target of rapamycin (mTOR) exists in two complexes to regulate diverse biological processes. Although mTOR complex 1, the canonical target of rapamycin, has been studied extensively in transplant settings, roles of mTORC2 remain relatively unknown. The aim of this study is to determine potentially distinctive roles of mTOR1 vs. mTOR2 in regulating liver ischemia-reperfusion injury (IRI). In a murine liver partial warm ischemia (90min.)/reperfusion (6h) model, we studies the activation of mTOR1 and mTOR2 by Western blot analysis of p70S6K and Akt (S473) phosphorylations, and compared therapeutic effects of Rapamycin (RPM) and Torin 1 (dual mTORC1 and 2 inhibitor) in liver IRI. In vitro, effects of selective knock-down of Raptor or Rictor (mTOR1 or 2 component, respectively) by their specific siRNA in hepatocytes on hepatocyte death against TNF-a were determined. Liver mTOR2 was constitutively active. Ischemia triggered a transient inactivation followed by a rapid reactivation during reperfusion (to the same level as in shams). Ischemia also inactivated mTOR1. A transient, but significantly enhanced, activation was triggered by reperfusion. Mice pre-treated with RPM (0.5-1mg/kg, i.p), but not Torin-1 (10mg/kg, i.p), were protected from IRI, as evidenced by lower sALT levels (Fig.) and better preserved liver architectures. RPM treatment inhibited mTORC1 activities, but enhanced liver mTORC2 activation during IR. The inhibition of Akt, downstream of mTORC2, by Triciribine (TCN, 1mg/kg) abolished liver protective effect of RPM (Fig.). In vitro, knock-down of Raptor reduced, while knock-down of Rictor increased, inflammatory hepatocyte death against TNF-a.. Thus, both mTOR complexes were involved in the pathogenesis of liver IRI by playing opposite roles in hepatocyte inflammatory response. Selective inhibition of mTORC1 with simultaneous activation of mTORC2 is critical for the hepatocytoprotection against IRI. 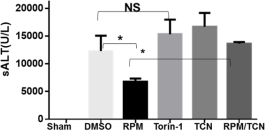
To cite this abstract in AMA style:
Zhou H, Zhu J, Yue S, Zhang M, Busuttil R, Xia Q, Kupiec-Weglinski J, Zhai Y. Distinctive Roles of mTORC1 Vs. mTORC2 in Liver Ischemia-Reperfusion Injury [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/distinctive-roles-of-mtorc1-vs-mtorc2-in-liver-ischemia-reperfusion-injury/. Accessed February 23, 2026.« Back to 2015 American Transplant Congress
