Tracking Tregs in a Non-Human Primate Model with Expanded Autologous Treg Infusions In Vivo
1Columbia Center for Translational Immunology, Columbia University, New York City, NY, 2Institute for Comparative Medicine, Columbia University, New York City, NY, 3Department of Pathology and Cell Biology, Columbia University, New York City, NY
Meeting: 2020 American Transplant Congress
Abstract number: D-301
Session Information
Session Name: Poster Session D: Cellular Therapies, Tissue Engineering / Regenerative Medicine
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
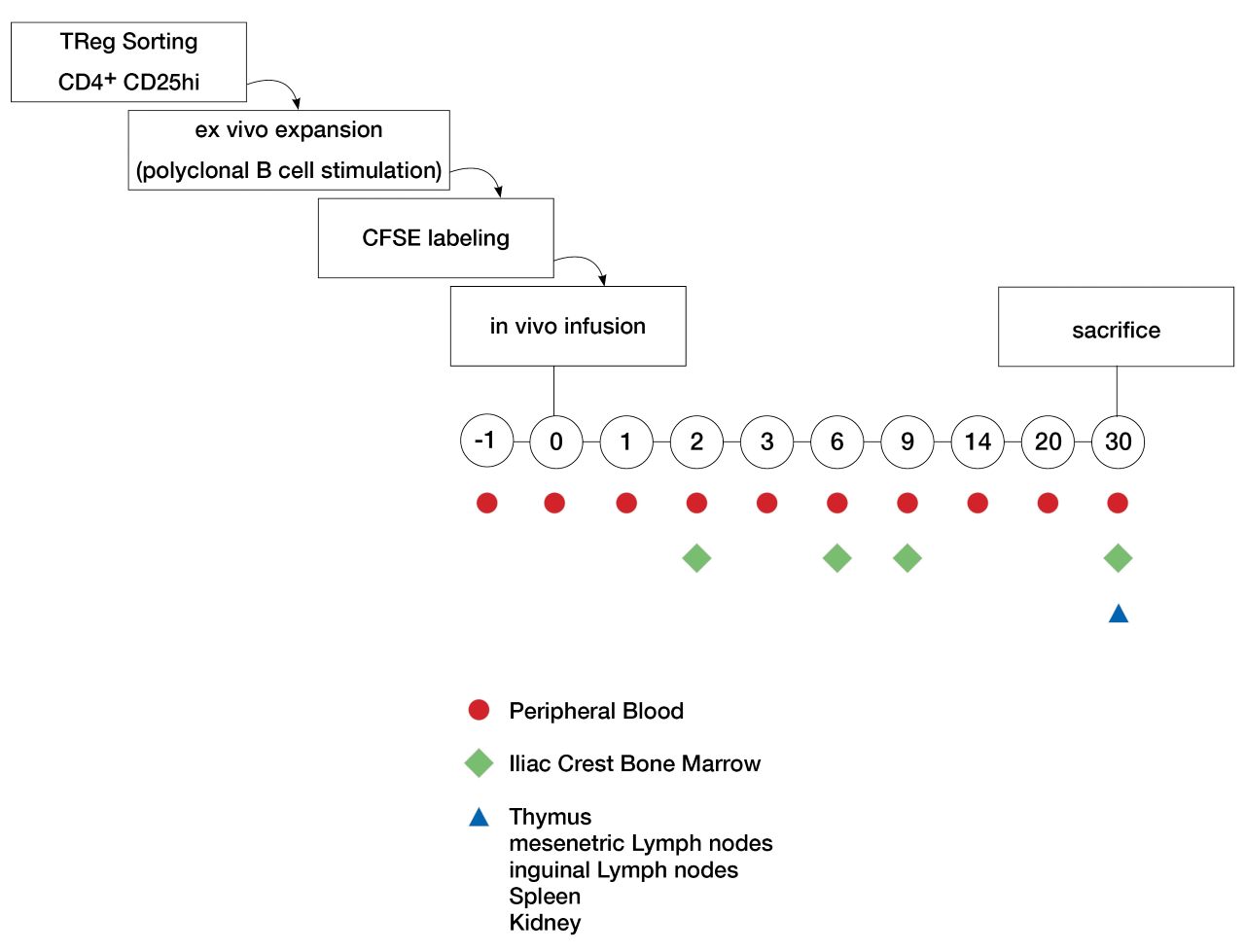
*Purpose: Regulatory Tcell (Treg) therapy is a promising tool for tolerance induction in solid organ transplantation. Despite encouraging results in humans and monkeys, questions about Treg phenotype, function, survival and migration after in vivo infusion remain. To better understand Treg fate, we have used a CFSE-labeled tracking method for autologous, polyclonally expanded Tregs in cynomolgus macaques.
*Methods: Polyclonal Tregs were grown from sorted (CD4+ CD25hi) PBMCs and ex vivo expanded using a B-cell expansion protocol and cryopreserved. 10-30×106 Tregs/kg were thawed and labeled with CFSE dye, then Tregs were administered intravenously. Phenotype and function of Tregs were specified via flow cytometry and suppression assays prior to in vivo infusion into 5 recipient cynomolgous macaques. Labeled Tregs were then tracked in vivo with flow cytometry on peripheral blood and iliac crest bone marrow aspirates over the first month. At the time of sacrifice (day 30), lymhoid organs (thymus, mesenteric and inguinal lymph nodes, spleen), kidney and bone marrow were harvested and analyzed.
*Results: The first cynomolgus macaque received 12×106 CFSE- labeled Tregs/kg that retained their phenotype (CD4+CD25hi, FoxP3+ expression ≥95%) and suppressive capacities (1:16 suppression titer) throughout the expansion and restimulation process. CFSE-labeled Tregs were detected in circulation at day 0, 2, 3, 6, 9 and 14 and were undetectable by day 15 after infusion. Dividing CFSE-labeled Tregs were seen at day 2, 6 and 9 in the bone marrow. At day 30, no CFSE-labeled cells were found in any tissues. Treg infusions are upcoming in 4 more cynomolgus macaques.
*Conclusions: CFSE labeling of autologous, ex vivo expanded polyclonal Tregs is a valid model that allows for in vivo tracking of migration and survival of Treg infusions in cynomolgus macaques.
To cite this abstract in AMA style:
Bruestle K, Piegari B, Sakai H, Fredriksson F, Ekanayake-Alper D, Robertson S, Hajosi D, Weiner J, Iuga A, Coley SM, Sykes M, Griesemer A. Tracking Tregs in a Non-Human Primate Model with Expanded Autologous Treg Infusions In Vivo [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/tracking-tregs-in-a-non-human-primate-model-with-expanded-autologous-treg-infusions-in-vivo/. Accessed January 16, 2026.« Back to 2020 American Transplant Congress