A CMV Vaccine Based on Non-Replicating Lymphocytic Choriomeningitis Virus Vectors Expressing gB and pp65 is Safe and Immunogenic in Healthy Volunteers and Entering a Phase 2 Trial in Kidney Transplant Recipients
C. N. Kotton1, M. Schwendinger2, G. Thiry3, B. De Vos4, F. De Boever5, G. Leroux-Roels6, A. Lilja7
1Infectious Diseases Division, Boston, MA, 2Hookipa Biotech AG, Vienna, Austria, 3Hookipa Biotech GmbH, VIenna, Austria, 4Bejamad sprl/bvba, Beersel, Belgium, 5Centrum voor vaccinologie (CEVAC), Gdent, Belgium, 6Centrum voor vaccinologie (CEVAC), Gent, Belgium, 7Hookipa Biotech GmbH, Vienna, Austria
Meeting: 2019 American Transplant Congress
Abstract number: 330
Keywords: Cytomeglovirus, Preclinical trails, Vaccination, Viral therapy
Session Information
Session Name: Concurrent Session: Breakthroughs in Cytomegalovirus
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Ballroom A
*Purpose: Cytomegalovirus (CMV) is a significant pathogen in pregnancy and immunocompromised patients. Antiviral prophylaxis is limited by toxicities, recurrent infection, and resistance. A safe and protective CMV vaccine is highly desirable.
*Methods: HB-101, a CMV vaccine, consists of non-replicating lymphocytic choriomeningitis virus vectors, expressing either the CMV tegument protein pp65 or a truncated isoform of the fusion protein gB. The safety and immunogenicity of HB-101 were evaluated in a randomized, placebo-controlled, double-blind phase I trial (NCT02798692). Three cohorts (1: 2.6×106; 2: 2.6×107and 3: 2.6 x 108FFU)of 18 subjects each were enrolled. On day 0, month 1, and month 3, HB-101 or placebo was administered to 14 and 4 subjects, respectively. Humoral and cellular responses against gB and the LCMV vector and cellular responses against pp65 were measured.
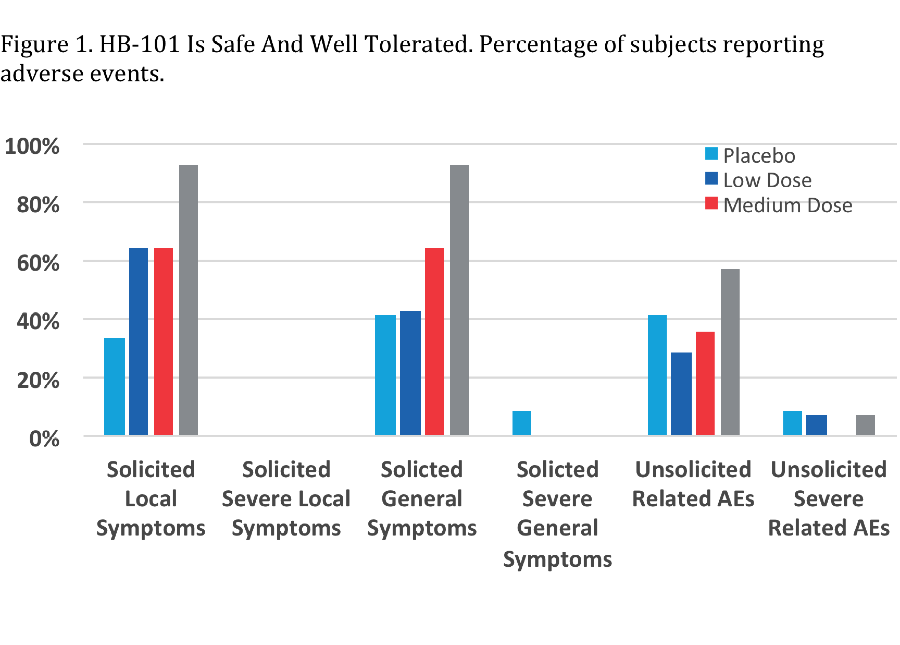
*Results: Injection site pain was the most frequent solicited adverse event (SAE)(Figure 1). Among the general SAE, malaise, fatigue and myalgia were most frequently reported. HB-101 induced gB-specific dose-dependent IgG antibody responses (Figure 2). All doses induced neutralizing antibodies and a robust, boosterable, durable T-cell response by IFNγ ELISPOT against CMV gB and pp65. Polychromatic flow cytometry indicated induction of a high proportion of polyfunctional HCMV-specific CD8 and CD4 T-cells. CD8 T-cells expressing IFNγ, IL2 and TNFα without CD107a were among the most prominent populations induced against CMV pp65.
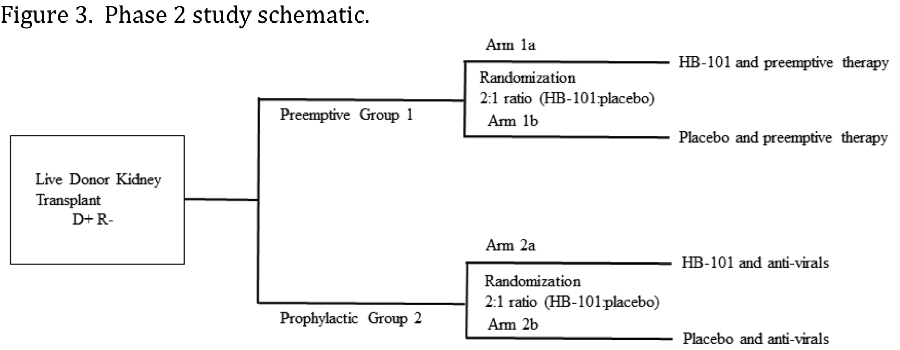
*Conclusions: A novel CMV vaccine with a good safety profile in healthy volunteers, HB-101 elicits strong humoral and cellular immune responses. We are enrolling a phase 2 trial in living donor kidney transplant recipients at high risk for CMV infection, with 2-3 doses of vaccine given prior to transplant (Figure 3) (NCT03629080).
To cite this abstract in AMA style:
Kotton CN, Schwendinger M, Thiry G, Vos BDe, Boever FDe, Leroux-Roels G, Lilja A. A CMV Vaccine Based on Non-Replicating Lymphocytic Choriomeningitis Virus Vectors Expressing gB and pp65 is Safe and Immunogenic in Healthy Volunteers and Entering a Phase 2 Trial in Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/a-cmv-vaccine-based-on-non-replicating-lymphocytic-choriomeningitis-virus-vectors-expressing-gb-and-pp65-is-safe-and-immunogenic-in-healthy-volunteers-and-entering-a-phase-2-trial-in-kidney-transplant/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress