An Improved Flow Cytometry Based Platelet Counting in Xeno Perfusion Models.
1Department of Surgery, University of Maryland School of Medicine, Baltimore, MD
2VA Medical Center, Baltimore, MD.
Meeting: 2016 American Transplant Congress
Abstract number: C131
Keywords: FACS analysis, Pig, Thrombocytopenia, Xenotransplantation
Session Information
Session Name: Poster Session C: Ischemia Reperfusion Injury and Organ Preservation
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Aim: Human or baboon platelet sequestration and activation occurs during pig organ xenograft perfusion; its mechanism remains poorly understood. In the context of a regimen where “rebound” of platelet counts was inconsistently but repeatedly observed, we evaluated and refined flow cytometry (FC) techniques in xenogenic perfusion models.
Methods: Heparin, anti-GpIb (2 lungs, 1 liver) and anti-CD18 (1 lung) treated human whole blood perfused through GalKO.hCD46.hCD55.hEPCR.hTFPI.hCD47 transgenic pig lungs (n=5) and livers (n=3) was analyzed using 3 hemocytometers (Hemavet, Advia 120, UniCel DxH 800), and a refined version of FC methods by ICSH (2001) and the BEST collaborative (2012), which avoid centrifugation and washing steps. FC data analyzed by FlowJo were compared with CBCs from hemocytometers.
Results: Mean of platelet counts measured by FC showed a decreasing trend over time in both lung and liver perfusions (Figure). On the other hand CBC data showed inconsistencies in- and inter-machine rerun of the same sample concordant with “rebound” observed in some xeno groups. Platelet counts from CBCs showed an increasing trend and large variations towards the end of the experiments (4 hours)(Figure). During FC analysis we observed an increasing population of events which were very close to platelets in size. These cells were not stained with both platelet markers (CD41, CD61); the majority of them were expressing a red blood cell marker (CD235a).
Conclusions: A flow cytometry method appears to offer improved accuracy and reproducibility relative to conventional hemocytometers which can give unreliable or unexpected results. Cause(s) of the apparent artifact yielding artificially high platelet counts as measured in clinical assays remains under active study. As supported by FC, falsely detected red blood cell fragments by hemocytometers can be one of the culprits cause pseudo-high platelet counts.
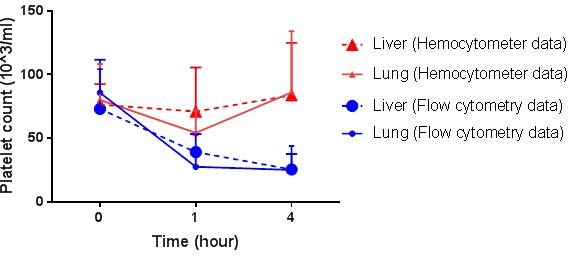 Figure. Comparison of platelet counts (mean±SD) from FC vs hemocytometers in ex-vivo xenogenic organ perfusions.
Figure. Comparison of platelet counts (mean±SD) from FC vs hemocytometers in ex-vivo xenogenic organ perfusions.
CITATION INFORMATION: Sendil S, Braileanu G, Sun W, Hassanein W, Parsell D, Crum H, Burdorf L, Pierson R, Azimzadeh A. An Improved Flow Cytometry Based Platelet Counting in Xeno Perfusion Models. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Sendil S, Braileanu G, Sun W, Hassanein W, Parsell D, Crum H, Burdorf L, Pierson R, Azimzadeh A. An Improved Flow Cytometry Based Platelet Counting in Xeno Perfusion Models. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/an-improved-flow-cytometry-based-platelet-counting-in-xeno-perfusion-models/. Accessed December 16, 2025.« Back to 2016 American Transplant Congress
