Tolerance to ABO A-Antigen Following Treatment of Infant Mice with A-Expressing MHC-Identical Erythrocytes
1Dept Pediatrics, Alberta Transplant Institute, Canadian Donation and Transplant Research Program, University of Alberta, Edmonton, AB, Canada, 2Department of Chemistry, University of Alberta, Edmonton, AB, Canada, 3Immunology Research Centre, St Vincent's Hospital, Melbourne, Australia
Meeting: 2019 American Transplant Congress
Abstract number: D56
Keywords: Antibodies, B cells, Heart, Lymphocytes
Session Information
Session Name: Poster Session D: Tolerance / Immune Deviation
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
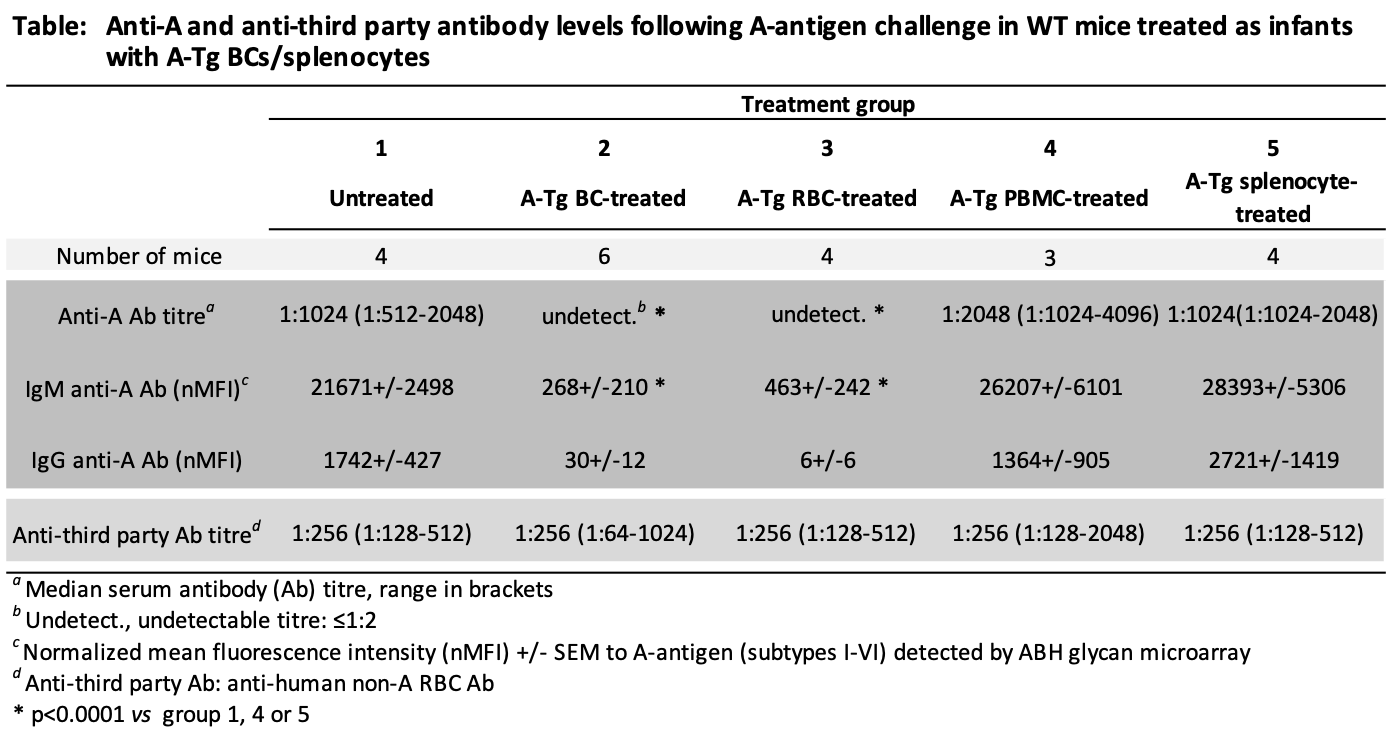
*Purpose: ABO-incompatible heart transplantation (ABOi HTx) is safe in young children and increases donor access. Post-ABOi HTx, B cell tolerance develops to donor ABO blood group antigen(s) by mechanisms not fully defined. To study ABO tolerance, we developed A-transgenic mice (A-Tg) that express A-antigen on vascular endothelium, erythrocytes (RBCs) and lymphocytes; we demonstrated A-antigen-specific tolerance induced by HTx into 4-week-old, MHC-identical, wild-type mice. Intentional induction of tolerance may allow subsequent ABOi HTx. Recently, we showed ABO tolerance could be intentionally induced in wild-type mice after intraperitoneal (ip) injection of infant mice with intact A-Tg blood cells (BCs). Herein, we sought to determine specific A-Tg cell type(s) capable of inducing tolerance.
*Methods: Wild-type BALB/c (BALB) mice at 7 days of age were left untreated or injected ip (weekly×3) with either unfractionated BCs, RBCs, peripheral blood mononuclear cells (PBMCs), or splenocytes from A-Tg BALB mice (see Table). Two weeks later, all mice were injected ip (weekly×5) with human A-RBC (A-antigen challenge) in an attempt to elicit anti-A antibody production. Serum anti-A and third-party (non-A anti-human) antibody were assessed by hemagglutination or ABH glycan microarray.
*Results: In response to challenge with A-antigen, high levels of anti-A antibody were produced both in untreated mice and in mice previously treated with A-Tg PBMCs or splenocytes (Table). In contrast, anti-A antibody remained undetectable/very low in mice treated as infants with A-Tg BCs or RBCs. Third-party antibody responses were high for all groups.
*Conclusions: A-Tg RBCs induced robust A-antigen-specific tolerance in infant mice, whereas A-Tg lymphocytes (PBMCs, splenocytes) did not. Future studies will explore mechanisms of A-Tg RBC tolerance induction with the goal to design synthetic ABH-multivalent polyethylene glycol (PEG) glycoconjugates for intentional ABO tolerance to allow subsequent ABOi HTx safely.
To cite this abstract in AMA style:
Motyka B, Fersovich J, Sulzer M, Adam I, Pearcey J, Tao K, Cairo CW, Cowan PJ, West LJ. Tolerance to ABO A-Antigen Following Treatment of Infant Mice with A-Expressing MHC-Identical Erythrocytes [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/tolerance-to-abo-a-antigen-following-treatment-of-infant-mice-with-a-expressing-mhc-identical-erythrocytes/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress