Tissue Engineering Scaffolds for Minimally Invasive Immunosurveillance of Acute Cellular Graft Rejection
1Biomedical Engineering, University of Michigan, Ann Arbor, MI, 2Internal Medicine, University of Michigan, Ann Arbor, MI, 3Human Genetics, University of Michigan, Ann Arbor, MI
Meeting: 2022 American Transplant Congress
Abstract number: 621
Keywords: Bioengineering, Non-invasive diagnosis, Protocol biopsy, Rejection
Topic: Basic Science » Basic Science » 02 - Acute Rejection
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
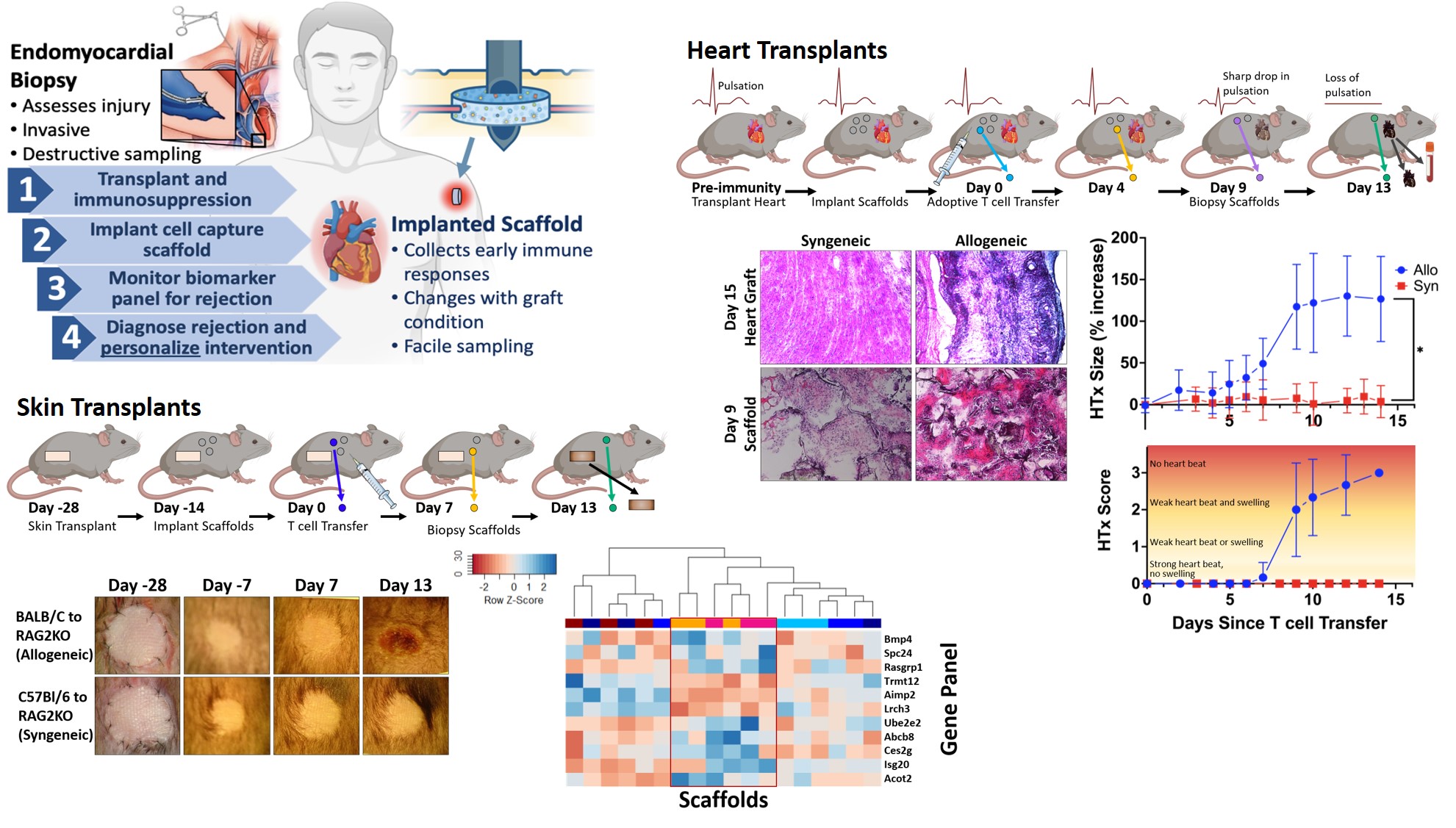
*Purpose: As there is no assay to predict the risk of acute cellular allograft rejection (ACAR), clinicians rely on protocol graft biopsy and aggressive, one-size-fits-all immunosuppression. Immunosuppression protects grafts from ACAR but increases systemic toxicities. Endomyocardial biopsy (EMB) histology suffers from variability and lags behind molecular biomarkers. A minimally invasive surveillance method is urgently needed to quantify the early risk of rejection for minimizing graft biopsy and personalizing treatment.
*Methods: Biomaterial scaffolds accumulate immune cells producing biomarkers of ACAR as an engineered immunological niche. We implanted subcutaneous poly-caprolactone scaffolds in murine heart or skin transplants to determine if they could identify ACAR. Rag2KO mice received full MHC mismatch allografts or syngeneic controls, and ACAR was initiated as a tunable event by C57BL/6 T cell transfer. Scaffolds were explanted and analyzed via histology and differential gene expression by RNA sequencing.
*Results: The scaffolds provided a novel measure of graft health. We algorithmically identified 11 differentially-expressed genes as biomarkers that distinguish mice with rejecting grafts from those with healthy grafts. Importantly, the scoring system detects oncoming ACAR prior to clinical signs of rejection. Also, the histology of these niches identified changes associated with ACAR progression.
*Conclusions: Gene expression in the cell-capture scaffold identified a biomarker signature of early ACAR, without invasive biopsy. Scaffold cell capture technology provides a leading indicator of ACAR by monitoring tissue-specific immune responses associated with allograft rejection. This implantable scaffold enables minimally invasive histological evaluation and molecular calculation of the early risk of rejection to reduce the frequency of routine EMB and personalize immune suppression that ultimately could prolong transplant life while minimizing risk.
To cite this abstract in AMA style:
Urie RR, Farris D, Morris A, Lombard E, Li JZ, Goldstein D, Shea L. Tissue Engineering Scaffolds for Minimally Invasive Immunosurveillance of Acute Cellular Graft Rejection [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/tissue-engineering-scaffolds-for-minimally-invasive-immunosurveillance-of-acute-cellular-graft-rejection/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress