The Polish Troubled Kidney Transplant Biopsy Study: External Validation of the Molecular Microscope System
1Pomeranian Medical University, Szczecin, Poland, 2Warsaw Medical Univeristy, Warsaw, Poland, 3Gdansk Medical University, Gdansk, Poland, 4Alberta Transplant Applied Genomics Centre, University of Alberta, Edmonton AB, AB, Canada, 5Medical University of Warsaw, Warsaw, Poland
Meeting: 2019 American Transplant Congress
Abstract number: A145
Keywords: Biopsy, Gene expression, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session A: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: The transplant clinician frequently encounters kidney transplant biopsies that are difficult to interpret by histology. A Molecular Microscope Diagnostic System (MMDx) has been developed in the INTERCOMEX study. We performed a prospective external validation study to determine how central MMDx assessment impacted the diagnosis and clinical decisions: the Polish Troubled Kidney Transplant Biopsies Study.
*Methods: Inclusion was based on clinician opinion that the biopsy was problematic. Four Polish transplant centers provided indication biopsies in accordance to local standard of care. 99 biopsies stabilized in RNA-later were transmitted at room temperature to the central laboratory in Canada and processed to generate an automated report, which was transmitted electronically to the clinicians within 48h (usually faster than histology report). We compared the results to local histology and assessed the agreement with clinician’s judgement and impact on clinical decisions.
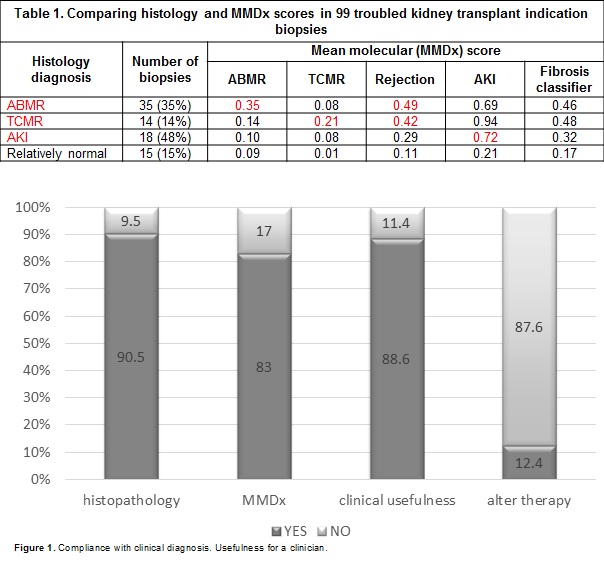
*Results: Biopsies were between 6 days and 20.2 years post-transplant. Rejection diagnoses by MMDx were ABMR (35%), TCMR (25%), mixed (2%) and AKI (32%). MMDx diagnoses correlated strongly with histologic diagnoses (Table 1). The molecular phenotype agreed with the conventional phenotype of TCMR (accuracy 64%) and ABMR (accuracy 81%), but with many disagreements. Comparison of conventional to central MMDx testing reveals crucial role of molecular tissue assessment in interpretation of BKV infection coexisting with tubulo-interstitial inflammation, i-IFTA, suspicious of rejection, proliferative arteriopathy and in reassuring treatment in doubtful cases of AR. In 90 feedback forms, clinicians indicated that 83% MMDx results agreed with clinical judgement; 88% indicated that MMDx invited additional confidence for management decisions and that in almost 13% of biopsies MMDx report would alter therapy (Figure 1).
*Conclusions: We conclude that real-time molecular assessment offers an important new tool for clinicians especially in interpreting troubled kidney transplant biopsies.
To cite this abstract in AMA style:
Myślak M, Deborska-Materkowska D, Ciszek M, Mazurkiewicz J, Debska-Slizien A, Halloran PF, Perkowska-Ptasińska A. The Polish Troubled Kidney Transplant Biopsy Study: External Validation of the Molecular Microscope System [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/the-polish-troubled-kidney-transplant-biopsy-study-external-validation-of-the-molecular-microscope-system/. Accessed March 7, 2026.« Back to 2019 American Transplant Congress