The Incidence of Polyomavirus-BK and Cytomegalovirus Infections in Renal Transplant Recipients Following Rituximab Administration
Northwestern Memorial Hospital, Chicago, IL.
Meeting: 2018 American Transplant Congress
Abstract number: 186
Keywords: B cells, CD20, Cytomeglovirus, Kidney transplantation
Session Information
Session Name: Concurrent Session: CMV: Bench to Bedside
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:06pm-3:18pm
Presentation Time: 3:06pm-3:18pm
Location: Room 608/609
Rituximab is an antibody that binds to the CD20 antigen on B cells resulting in rapid and prolonged depletion. At Northwestern Memorial Hospital, a single dose of rituximab is given to renal transplant recipients with documented historical donor specific antibodies (DSA). There is concern that the administration of rituximab with alemtuzumab induction may lead to increased risk of infectious complications.
Purpose
This study aims to determine if the administration of rituximab for historical DSA in renal transplant recipients who received alemtuzumab induction leads to an increased rate of polyomavirus-BK and cytomegalovirus (CMV) infectious complications.
Methods
Single center, retrospective cohort study of renal transplant recipients who received a single dose of rituximab for historical DSA with alemtuzumab induction. Patients ≥ 18 years old who were transplanted between January 1st 2007 and October 31st 2016 were included. These patients were matched 1:1 based on donor/recipient CMV serostatus with patients who did not receive rituximab. Incidence of polyomavirus-BK viruria, viremia, nephropathy and CMV viremia within one year post-transplant were evaluated.
Results
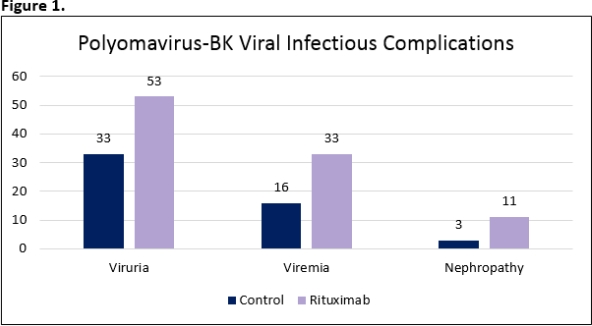
A total of 326 patients (163 patients in each group) were included in the study. Patient demographics are summarized in Table 1. Incidence of polyomavirus-BK viruria (p<0.01), viremia (p<0.01) and nephropathy (p=0.05) were significantly greater in the rituximab group compared to the control group (Figure 1). There was no difference in CMV viremia between the rituximab and control (p=0.31).
Conclusions
Rituximab administration for historical DSA in renal transplant recipients also receiving alemtuzumab induction was associated with higher rates of polyomavirus-BK infectious complications within one year post-transplant.
CITATION INFORMATION: Kraljevic A., D'Agostino C., Kane C., Cunningham K., Shetty A., Richardson C. The Incidence of Polyomavirus-BK and Cytomegalovirus Infections in Renal Transplant Recipients Following Rituximab Administration Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kraljevic A, D'Agostino C, Kane C, Cunningham K, Shetty A, Richardson C. The Incidence of Polyomavirus-BK and Cytomegalovirus Infections in Renal Transplant Recipients Following Rituximab Administration [abstract]. https://atcmeetingabstracts.com/abstract/the-incidence-of-polyomavirus-bk-and-cytomegalovirus-infections-in-renal-transplant-recipients-following-rituximab-administration/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress