The Effect of Rituximab on Hepatitis B Reactivation in Kidney Transplant Recipients with Resolved Hepatitis B.
1Surgery, Yonsei University College of Medicine, Seoul, Korea
2Internal Medicine (Gastroenterology), Yonsei University College of Medicine, Seoul, Korea
3Internal Medicine (Nephrology), Yonsei University College of Medicine, Seoul, Korea.
Meeting: 2016 American Transplant Congress
Abstract number: A105
Keywords: B cells, Hepatitis B, Kidney transplantation, Monoclonal antibodies
Session Information
Session Name: Poster Session A: Kidney Desensitization
Session Type: Poster Session
Date: Saturday, June 11, 2016
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Halls C&D
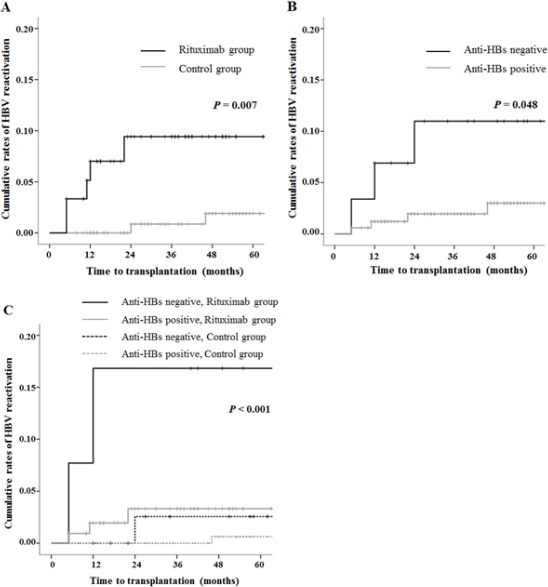
Background: Hepatitis B virus (HBV) reactivation is a well-known complication of immunosuppressive therapy in resolved HBV (hepatitis B surface antigen [HBsAg] negative and hepatitis B core antibody [anti-HBc] positive) patients. Although increasing use of rituximab in kidney transplantation, the risk of HBV reactivation after kidney transplantation remains undetermined.
Methods: We analyzed 199 resolved HBV patients who underwent living donor kidney transplantation between 2008 and 2014. The patients were divided into rituximab group (n=61) and control group (n=138). All patients were observed for HBV reactivation, which was defined as HBsAg seroreversion. No patients received prophylactic antiviral agents. Entecavir was started for the patients since HBV reactivation.
Results: During the follow-up period, five patients (8.2%) in rituximab group and two patients (1.4%) in control group developed HBV reactivation (p=0.007). All patients showed serum HBV DNA level of more than 7 log 10 IU/mL. In rituximab group, 4 patients experienced HBV-related hepatitis flare (serum alanine aminotransferase more than 100 IU/L), including one patient who died of hepatic failure. The median time from the rituximab treatment to HBV reactivation was 11 months (range, 5 – 22). In contrast, one patient experienced hepatitis flare in control group. Use of rituximab (hazard ratio, 7.84; 95% CI, 1.50 to 40.00; p= 0.015) and hepatitis B surface antibody status (hazard ratio, 4.97; 95% CI, 1.11 to 22.34, p=0.037) were the significant risk factors for the HBV reactivation.
Conclusion: Rituximab significantly increased the risk of HBV reactivation in resolved HBV patients after kidney transplantation. Close monitoring and prophylaxis strategies are required in these patients.
CITATION INFORMATION: Lee J, Park J, Lee J, Song S, Lee J, Kwon S.-K, Huh K, Kim B, Kim M, Kim S, Ahn S, Kim Y. The Effect of Rituximab on Hepatitis B Reactivation in Kidney Transplant Recipients with Resolved Hepatitis B. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Lee J, Park J, Lee J, Song S, Lee J, Kwon S-K, Huh K, Kim B, Kim M, Kim S, Ahn S, Kim Y. The Effect of Rituximab on Hepatitis B Reactivation in Kidney Transplant Recipients with Resolved Hepatitis B. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/the-effect-of-rituximab-on-hepatitis-b-reactivation-in-kidney-transplant-recipients-with-resolved-hepatitis-b/. Accessed July 18, 2025.« Back to 2016 American Transplant Congress
