Targeted Rapamycin Nanotherapy Dampens Memory T Cell Function
1Surgery, Microbiology and Immunology, Medical University of South Carolina, Charleston, SC
2Radiologic Sciences, Medical University of South Carolina, Charleston, SC
3Microbiology and Immunology, Medical University of South Carolina, Charleston, SC.
Meeting: 2015 American Transplant Congress
Abstract number: A268
Keywords: Bioengineering, Interferon (IFN), Rapamycin, T cells
Session Information
Session Name: Poster Session A: Preclinical Immunosuppression and Tolerance
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Memory T cells (Tm) are difficult to control post-transplantation and account for early rejection episodes. Additionally, therapies exerting local immunosuppression by targeting an allograft, while reducing side-effects offer great potential. Novel Targeted Rapamycin Micelle (TRaM) nanoparticles with pH-triggered release of rapamycin at endothelial cells were used to assess Tm function when compared to naïve effector cells in varying oxygen tension environments. 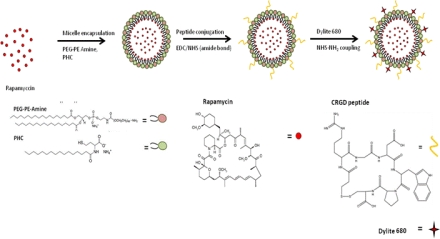
Methods: TRaMs were conjugated to fluorophores and arginine-glycine-aspartate for tracking and targeting to α v β 3 integrins. Mouse cardiac endothelial cells (MCEC) from FVB mice were inoculated into allogeneic C57/BL6 mice for 21 days. Splenic Tm of host mice were cultured with MCEC for 72 hours. As a control, splenic T cells from naïve C57/BL6 mice were cultured with MCEC. Cultures were exposed to both a hypoxic chamber, mimicking organ ischemia, and normoxic conditions. ELISA for IFN-γ was performed on culture supernatants.
Results: Tm produced marked amounts of IFN-γ when cultured with MCECs in hypoxic (40.54 +/- 3.38 pg/ml) and normoxic (83.76 +/- 9.8 pg/ml) conditions. Tm production of IFN-γ was significantly reduced when cultures were treated with TRaM in hypoxic and normoxic settings (17.95 +/- 1.389 pg/ml and 32.70 +/- 6.929 pg/ml; p ≤ 0.0001.) Cultures treated with TRaM showed a therapeutic effect similar to free rapamycin. 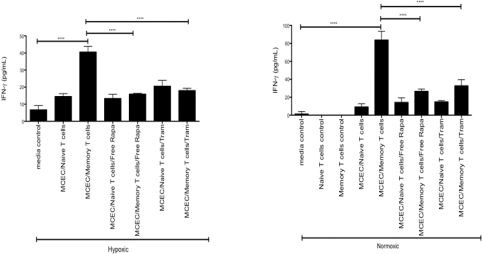
Conclusions: TRaMs have proven beneficial as a local immunosuppressive device. These data support the ability of TRaM to also blunt the function of Tm in the face of ischemia as well as normal oxygen tension.
To cite this abstract in AMA style:
Nadig S, Esckilsen S, Broome A-M, Dixit S, Atkinson C. Targeted Rapamycin Nanotherapy Dampens Memory T Cell Function [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/targeted-rapamycin-nanotherapy-dampens-memory-t-cell-function/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
