Subclinical Histologic Findings Are Observed in 25% of Stable Adult Liver Transplant Recipients (ALTRs) Screened for Immunosuppression Withdrawal (ISW): OPTIMAL (NCT02533180).
1ITN, San Francisco
2Northwestern, Chicago
3UCSF, San Francisco
4UPMC, Pittsburgh
5Columbia, New York
6Baylor, Dallas
7Rho, Chapel Hill
8NIAID/NIH, Bethesda
9ITN, Bethesda
10MGH, Boston
Meeting: 2017 American Transplant Congress
Abstract number: D204
Keywords: Histology, Immunosuppression, Liver transplantation, Tolerance
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
OPTIMAL is a 6-center ISW study in non-autoimmune, non-HCV ALTRs ≥3 yrs post-transplant (tx). Studies have shown a correlation between portal inflammation associated with tissue damage and ISW failure, so subjects had a liver biopsy prior to ISW to exclude unfavorable histology and identify tolerance biomarkers.
Methods: 44 screening biopsies were performed 12/15–11/16. Histologic inclusion criteria are noted in Table 1. Histologically eligible/ineligible subjects were compared using Fisher's exact or Wilcoxon rank sum tests with significance at α ≤0.05.
Results: 11/44 subjects were ineligible due to inflammation (n=8) and/or fibrosis (n=3), or bile duct damage (n=1) (Table 2). No significant differences were observed between eligible/ineligible subjects regarding cause of liver failure/blood type/sex/race, IS type, time post-tx, ALT/GGT, or donor type/age/sex. Eligible subjects were significantly older at tx (55 vs. 47, p=0.02) and enrollment (63 vs. 51, p=<0.01).
Conclusion: 1 in 4 biopsies from long-term, stable, non-autoimmune, non-HCV ALTRs harbored subclinical inflammation and/or fibrosis. Increased recipient age at tx and enrollment, but not time from tx, correlated with histologic eligibility for ISW. The natural history and significance of these biopsy findings is unknown and merits further study.
Table 1. Screening Biopsy Inclusion Criteria
| Absent/mild | Portal inflammation |
| Interface inflammation | |
| Fibrosis | |
| Absent | Centrizonal/perivenular inflammation |
| Arteriopathy | |
| Bile duct changes (damage, ductopenia, or biliary epithelial senescence) |
Table 2. Characteristics of Eligible/Ineligible Subjects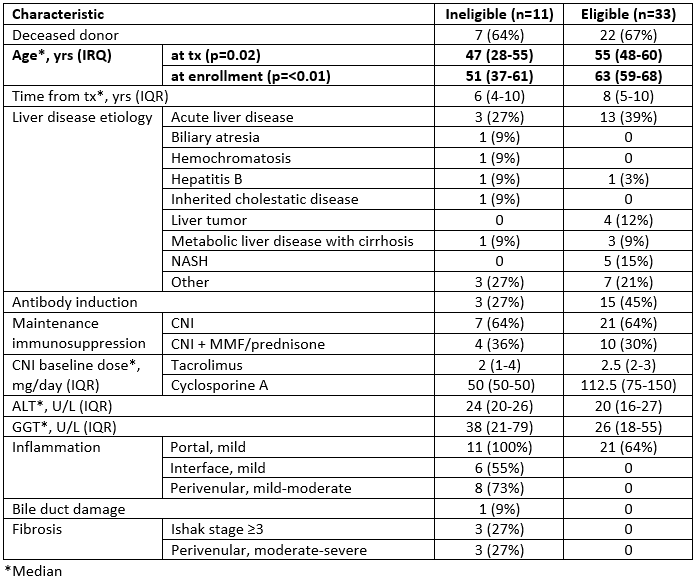
CITATION INFORMATION: Chandran S, Abecassis M, Levitsky J, Feng S, Humar A, Emond J, Klintmalm G, Demetris A, Much K, Sun L, Priore A, Ikle D, Bridges N, DesMarais M, Burrell B, Markmann J. Subclinical Histologic Findings Are Observed in 25% of Stable Adult Liver Transplant Recipients (ALTRs) Screened for Immunosuppression Withdrawal (ISW): OPTIMAL (NCT02533180). Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Chandran S, Abecassis M, Levitsky J, Feng S, Humar A, Emond J, Klintmalm G, Demetris A, Much K, Sun L, Priore A, Ikle D, Bridges N, DesMarais M, Burrell B, Markmann J. Subclinical Histologic Findings Are Observed in 25% of Stable Adult Liver Transplant Recipients (ALTRs) Screened for Immunosuppression Withdrawal (ISW): OPTIMAL (NCT02533180). [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/subclinical-histologic-findings-are-observed-in-25-of-stable-adult-liver-transplant-recipients-altrs-screened-for-immunosuppression-withdrawal-isw-optimal-nct02533180/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
