Single-Dose Basiliximab Induction in Liver Transplant Recipients with Delayed CNI Initiation
1Pharmacy, NewYork-Presbyterian Hospital, New York, NY
2Medicine, Weill Cornell Medicine, New York, NY
3Pharmacy, Hospital of the University of Pennsylvania, Philadelphia, PA.
Meeting: 2018 American Transplant Congress
Abstract number: D214
Keywords: Calcineurin, Induction therapy, Liver transplantation, Rejection
Session Information
Session Name: Poster Session D: Liver - Kidney Issues in Liver Transplantation
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Liver transplant recipients (LTR) with peri-operative renal injury benefit from a delayed CNI protocol using basiliximab (IL2RA) induction to reduce early CNI exposure. Existing literature in renal transplant demonstrates comparable efficacy among recipients of single-dose vs. two doses of IL2RA. The objective was to compare the rates of BPAR among LTR who received one dose vs. two doses of IL2RA using the delayed CNI protocol.
All adult LTR between 01/07 and 12/15 who received IL2RA induction were retrospectively assessed. LTR undergoing retransplant, dual organ transplant, and those who died within 90 days were excluded. All LTR followed the same immunosuppression maintenance protocol with delayed CNI initiation. The primary outcomes were the observed 30-day and 90-day rates of BPAR between the two groups (one vs. two dose) and multivariable logistic regression was used to control for predictors of BPAR.
A total of 292 LTR were included in the analysis; 11 patients received one dose of IL2RA compared to 281 LTR who received two doses. 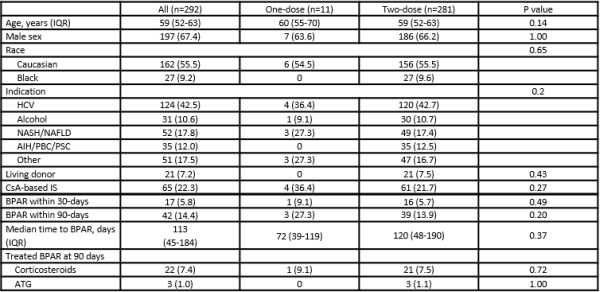 The majority of LTR were Caucasian (56%), male (67%), and had a median age of 59 years. Baseline demographics were similar between groups. There were no significant differences in BPAR rates between the one-dose and two-dose groups at either 30 days (9% vs. 6%; p=0.5) or 90 days (27% vs. 14%; p=0.2). On multivariable analysis, use of one-dose IL2RA was not predictive for 30-day BPAR (OR 1.6, 95% CI 0.2–13.5, p=0.7) or 90-day BPAR (OR 2.3, 95% CI 0.6–9.2, p=0.2), while only recipient Hispanic race (OR 4.2; 95% CI 1.4–12.8; p=0.01) was significant for BPAR at 30 days. There were also no differences in median time to a BPAR event (72 vs. 120 days; p=0.4) or treated BPAR (p=0.7).
The majority of LTR were Caucasian (56%), male (67%), and had a median age of 59 years. Baseline demographics were similar between groups. There were no significant differences in BPAR rates between the one-dose and two-dose groups at either 30 days (9% vs. 6%; p=0.5) or 90 days (27% vs. 14%; p=0.2). On multivariable analysis, use of one-dose IL2RA was not predictive for 30-day BPAR (OR 1.6, 95% CI 0.2–13.5, p=0.7) or 90-day BPAR (OR 2.3, 95% CI 0.6–9.2, p=0.2), while only recipient Hispanic race (OR 4.2; 95% CI 1.4–12.8; p=0.01) was significant for BPAR at 30 days. There were also no differences in median time to a BPAR event (72 vs. 120 days; p=0.4) or treated BPAR (p=0.7).
There were no significant differences in the incidence of BPAR, time to BPAR, or treated BPAR between patients who received one-dose vs. two-dose of IL2RA. However, further investigation with longer follow up on the use of one-dose IL2RA among highly selected patients is warranted.
CITATION INFORMATION: Salerno D., Lange N., Rosenblatt R., Sammons C., Fortune B. Single-Dose Basiliximab Induction in Liver Transplant Recipients with Delayed CNI Initiation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Salerno D, Lange N, Rosenblatt R, Sammons C, Fortune B. Single-Dose Basiliximab Induction in Liver Transplant Recipients with Delayed CNI Initiation [abstract]. https://atcmeetingabstracts.com/abstract/single-dose-basiliximab-induction-in-liver-transplant-recipients-with-delayed-cni-initiation/. Accessed February 22, 2026.« Back to 2018 American Transplant Congress
