Selective Deletion of Notch2 on B Cells Abrogates Alloantibody Production in Fully MHC-Mismatched Cardiac and Skin Transplant Models.
Schuster Transplantation Research Center, Brigham & Women's Hospital, Harvard Medical School, Boston, MA.
Meeting: 2016 American Transplant Congress
Abstract number: 470
Keywords: Alloantibodies, B cells, Co-stimulation, Lymphocytes
Session Information
Session Name: Concurrent Session: B cells and Antibody-Mediated Rejection: Animal Models
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 309
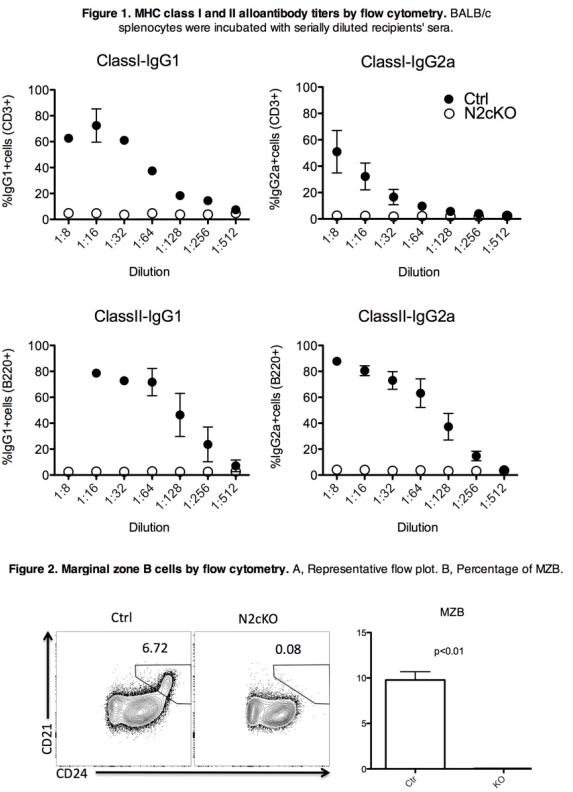
Alloantibody-mediated injury remains a major challenge for the improvement of long-term graft survival and there is no effective therapy to prevent alloantibody generation post-transplant. The Notch pathway is a major cell-cell signaling pathway that plays a crucial role in cell development and fate. In the immune system, Notch2 is differentially expressed on B cell subsets and is known to be critical for marginal zone B cells (MZB) differentiation and positioning in secondary lymphoid organs. However, little is known about the role of Notch2 in alloantibody production post-transplantation.
Herein, using a fully MHC-mismatch cardiac and skin transplant model (BALB/c into C57BL/6J), we report that Notch2fl/flCD19-Cre (Notch2cKO) recipients do not develop allo-specific antibodies both short- and long-term (day 8 and >120 after transplant, respectively) . This was consistent with our previous findings that transient administration of anti-Notch2 antibody (day 0, 3, 5, 7, 9, 11) in a similar cardiac transplant model completely suppressed alloantibody production. Interestingly, this attenuation of alloantibody production in Notch2cKO mice is associated with less plasma cells (B220loCD138+; 0.038±0.16% vs. 0.34±0.02 on controls, p<0.01), germinal center cells (GL7+Fas+; 0.27±0.054% vs 1.1±0.25, p=0.19), T follicular helper cells (Tfh; CD4+PD1+CXCR5+; 4.66±0.29% vs 5.83±0.26, p=0.022) and MZB cells (Figure below).
These data indicate that Notch2 signaling is critical for the alloantibody generation post-transplantation and suggest that selective Notch2 blockade might be a promising therapeutic strategy to prevent the antibody-mediated injury so resistant to current therapy.
CITATION INFORMATION: Murakami N, Magee C, Borges T, Azzi J, Riella L. Selective Deletion of Notch2 on B Cells Abrogates Alloantibody Production in Fully MHC-Mismatched Cardiac and Skin Transplant Models. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Murakami N, Magee C, Borges T, Azzi J, Riella L. Selective Deletion of Notch2 on B Cells Abrogates Alloantibody Production in Fully MHC-Mismatched Cardiac and Skin Transplant Models. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/selective-deletion-of-notch2-on-b-cells-abrogates-alloantibody-production-in-fully-mhc-mismatched-cardiac-and-skin-transplant-models/. Accessed February 21, 2026.« Back to 2016 American Transplant Congress
