Rituximab Interference in B-Cell Crossmatch: A Problem Solved
1Service de Néphrologie, Dialyse, Aphérèses et Transplantation, CHU Grenoble Alpes, Grenoble, France
2Laboratoire d'Histocompatibilité, EFS Rhone Alpes, Grenoble, France.
Meeting: 2018 American Transplant Congress
Abstract number: 542
Keywords: Antibodies, FACS analysis, Histocompatibility
Session Information
Session Name: Concurrent Session: Kidney: Perioperative Considerations
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 303
Background: Rituximab is commonly prescribed in ABO- or HLA-incompatible kidney transplantation. When used before transplant it interferes with B lymphocyte complement-dependent crossmatch (CDC CM) or FACS crossmatch (FACS CM) results thus rendering their interpretation impossible. This effect has been described up to 9 months following rituximab infusion.
We describe here a new method to abolish rituximab interference in both CDC CM and FACS CM.
Methods: We used an anti-rituximab mouse monoclonal antibody (10C5 clone, ABNOVA®) directed against the F(ab)2 fragment of rituximab. Normally this antibody is employed to dose rituximab by ELISA technique.
We tested different antibody concentrations in different settings (rituximab infusions 0 days to 270 days before CM; patients with and without class 1 and 2 HLA antibodies) and evaluated whether the interference of rituximab was abolished in FACS CM and CDC CM.
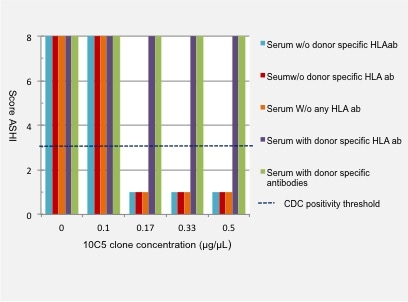
Results: A concentration of 0.17[micro]g/[micro]l of the 10C5 clone abolished completely false positive results in both CDC and FACS CM. 1[micro]l of 10C5 was thus added to 5[micro]l of serum for 15 minutes at room temperature, the CM was then performed. While these concentrations dilute the serum they did not nullify expected positive results. CDC and FACS CM stayed positive when donor specific HLA antibodies had a Luminex mean fluorescence intensity respectively >8000 and >4000.
Conclusion: We describe an easy, efficient and relatively inexpensive method to eliminate rituximab interference on CDC and FACS CM results.
CITATION INFORMATION: Malvezzi P., Senoussi O., Jouve T., De Palma C., Rostaing L., Bardy B., Masson D. Rituximab Interference in B-Cell Crossmatch: A Problem Solved Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Malvezzi P, Senoussi O, Jouve T, Palma CDe, Rostaing L, Bardy B, Masson D. Rituximab Interference in B-Cell Crossmatch: A Problem Solved [abstract]. https://atcmeetingabstracts.com/abstract/rituximab-interference-in-b-cell-crossmatch-a-problem-solved/. Accessed July 18, 2025.« Back to 2018 American Transplant Congress