Randomized Controlled Study Evaluating Adherence Monitoring with Electronic Feedback on Reducing Renal Allograft Rejection.
Transplant Center, University of Chicago, Chicago, IL.
Meeting: 2016 American Transplant Congress
Abstract number: 281
Session Information
Session Name: Concurrent Session: Antibody Mediated Rejection in Kidney Transplantation: De Novo DSA
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Ballroom B
Purpose: The negative impact of non-adherence to the immunosuppressant medication (IM) regimen on transplant outcomes is well recognized. In an attempt to improve outcomes, the SIMpill® System is currently being utilized in kidney transplant(KT) recipients in a 3-year study in which allograft rejection is being evaluated.
Methods: KT recipients(n=46) were randomized to 4 groups, 2 Intervention Groups(I1 & I2) or 2 Control Groups(C1 & C2). Subjects enrolled in I1, I2 and C1 received the SIMpill® Electronic Medication Adherence Monitoring System. C2 did not receive a device. This system is a medication-dispensing device that communicates and stores timing of openings, or doses taken to a secure server. The Intervention Group subjects received notification if a scheduled IM dose was missed via text message and/or email. In addition, in I2, if a scheduled dose was missed despite the reminder message, a study provider was notified, enabling intervention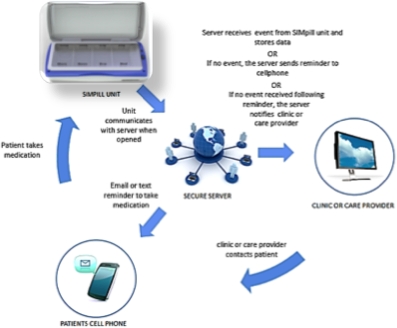 . In C1 no one received any feedback, but their adherence is stored on the server for analysis.
. In C1 no one received any feedback, but their adherence is stored on the server for analysis.
Results: At one year, 46 KT recipients were enrolled with an average follow-up of 334 days (median=413.5[range 118-559]). Seven of 9 rejections were ACR where 2 of the 9 were ABMR. Five of 7 ACR biopsies were categorized as Banff grade ≤ 2 while the other 2 were classified as borderline. Five of 9 rejection episodes resulted in hospital admission totaling 34 days. One of the 9 subjects with ACR experienced graft failure and died on post-transplant day 140
| Adherence Parameter | Intervention Group 1 & 2 N=20 | Control Group 1 N=26 | p-value |
| # of Biopsies performed | 4 | 9 | p=NS |
| Biopsy Proven Rejection(% of group) | 0(0) | 9(19.2) | <0.005 |
| LOS for treatment days | 0 | 34 | <0.001 |
| Total Doses Taken % | 90 | 84 | <0.03 |
| Days with Correct Dosing % | 87 | 74 | <0.02 |
.
Conclusions: Preliminary 1-year results demonstrate that utilization of the SIMpill® with feedback on adherence shows a potential reduction in biopsies performed, and rejection episodes as well possibly reducing hospitalizations and cost related to rejection.
CITATION INFORMATION: Bodzin A, Becker Y, Josephson M, Witkowski P, Lockwood M, Potter L, Kane B, Lourenco L, Millis J. Randomized Controlled Study Evaluating Adherence Monitoring with Electronic Feedback on Reducing Renal Allograft Rejection. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Bodzin A, Becker Y, Josephson M, Witkowski P, Lockwood M, Potter L, Kane B, Lourenco L, Millis J. Randomized Controlled Study Evaluating Adherence Monitoring with Electronic Feedback on Reducing Renal Allograft Rejection. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/randomized-controlled-study-evaluating-adherence-monitoring-with-electronic-feedback-on-reducing-renal-allograft-rejection/. Accessed February 20, 2026.« Back to 2016 American Transplant Congress
