Proteomics Reveals Compartment-Specific Alterations in the Extracellular Matrix of Kidney Allografts with Antibody-Mediated Rejection
1University Health Network, Toronto, ON, Canada, 2Lunenfeld-Tanenbaum Research Institute, Toronto, ON, Canada, 3Krembil Research Institute, Toronto, ON, Canada, 4Institute of Medical Science, University of Toronto, Toronto, ON, Canada
Meeting: 2019 American Transplant Congress
Abstract number: 472
Keywords: Adhesion molecules, Antibodies, Biopsy, Major histocompatibility complex (MHC)
Session Information
Session Name: Concurrent Session: Biomarkers, Immune Monitoring and Outcomes IV
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 306
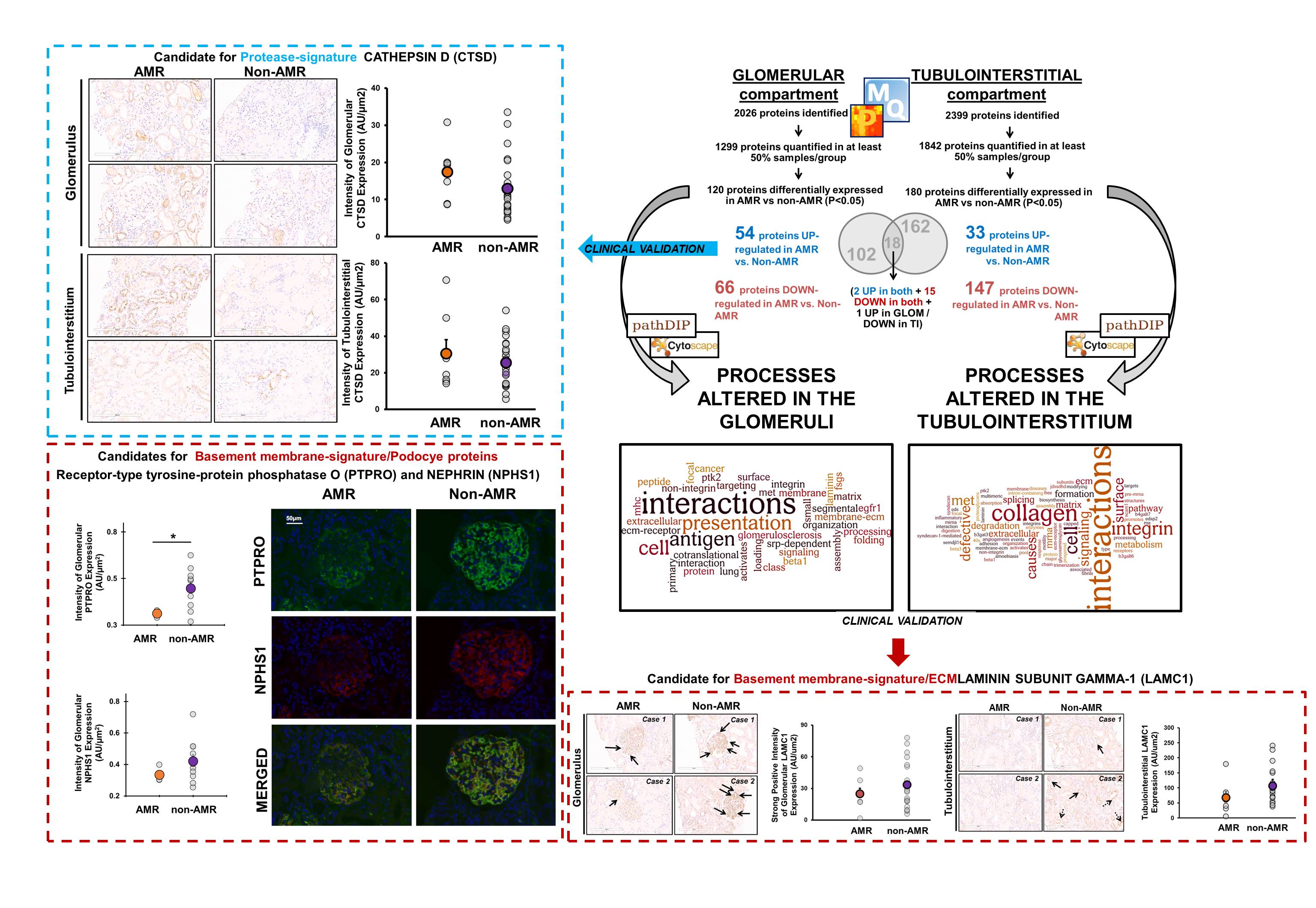
*Purpose: Antibody-mediated rejection (AMR) is the most important cause of premature kidney graft loss. AMR is caused by anti-HLA and non-HLA antibodies against proteins in two main kidney compartments: glomeruli and tubulointerstitium. We hypothesized that acute AMR is associated with compartment-specific proteome alterations that may uncover the mechanisms of early antibody-mediated injury.
*Methods: We isolated glomeruli and tubulointerstitium by laser capture-microdissection from FFPE kidney biopsies, and subjected samples to proteome analysis on a Q-Exactive-Plus mass spectrometer. We compared 7 biopsies with pure AMR with 23 matched ‘non-AMR’ biopsies with acute cellular rejection (ACR) or tubular necrosis (ATN). All biopsies were for-cause and scored using Banff classification (2017).
*Results: The median post-transplant graft age at the time of the biopsy was 11 days. AMR biopsies were c4d+ and no biopsies had chronic lesions. We identified 2026 proteins in glomeruli and 2399 in tubulointerstitium (FDR<0.01). 120 glomerular proteins and 180 tubulointerstitial proteins were differentially expressed (p<0.05) in AMR vs. non-AMR tissues. Proteins involved in HLA presentation were increased in both AMR compartments. Proteins significantly decreased in both compartments in AMR (e.g. LAMC1, COL1A1, NID1) belong to basement membranes and processes such as collagen, extracellular matrix (ECM) and cytoskeleton. We confirmed by immunochemistry lower protein expression of LAMC1, PTPRO and NPHS1, and higher levels of glomerular CTSD (a protease involved in collagen degradation and ECM remodeling) in AMR vs. non-AMR biopsies.
*Conclusions: Basement membranes are often remodeled in late chronic AMR. Thus, these proteomic changes may represent early, key alterations in AMR. Targeting early ECM remodeling in AMR may represent a new therapeutic opportunity.
To cite this abstract in AMA style:
Freixas SClotet, McEvoy C, Batruch I, Kotlyar M, Pastrello C, Van J, Bozovic A, Kulasingam V, Chen P, Jurisica I, Chruscinski A, John R, Konvalinka A. Proteomics Reveals Compartment-Specific Alterations in the Extracellular Matrix of Kidney Allografts with Antibody-Mediated Rejection [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/proteomics-reveals-compartment-specific-alterations-in-the-extracellular-matrix-of-kidney-allografts-with-antibody-mediated-rejection/. Accessed March 1, 2026.« Back to 2019 American Transplant Congress