Prognostic Tools to Choose Candidates for Successful Desensitization.
1Northwestern University, Chicago, IL
2Johns Hopkins, Baltimore, MD
Meeting: 2017 American Transplant Congress
Abstract number: A52
Keywords: HLA antibodies, IVIG, Kidney transplantation, Plasmapheresis
Session Information
Session Name: Poster Session A: Clinical Science: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Antibodies to donor HLA antigens are a barrier to solid organ transplantation. Strategies to desensitize patients have improved, but the ability to predict response to or monitor the efficacy of treatment is deficient. We previously reported that titration studies are a consistent and accurate means of assessing antibody strength. Here we tested pre- and post-desensitization samples from 31 patients at two centers using single antigen HLA Class I and II beads, and compared their responses to treatment. Desensitization therapy consisted of a single dose of rituximab (375 mg/m2) followed by a variable number of rounds of plasmapheresis + IVIg (100mg/kg).
In contrast to previous reports, we observed that the reduction in titer for both Class I and II antibodies was consistent and correlated with the number of rounds of treatment. Figure 1 shows an increase in mean Log2 reduction of antibody titer up to a 3.6 and 3.9 for Class I and II, respectively, by 7 rounds of treatment. The relative size of the circle indicates the density of data points for a given Log2-titer reduction (Y-axis) per treatment round (X-axis). Of note, reduction in titer plateaued with additional cycles, indicating a potential limit to the ability of this treatment to remove antibodies. Specifically, despite as many as 14 rounds of therapy, antibodies with an original titer > 1:1024 were not lowered to a level correlating with an expected negative flow crossmatch.
Using MFI values as point of reference did not correlate with success of treatment if MFI values in undiluted samples were >10,000. Beyond that point, correlation with titer value was very poor, likely due to saturation of the beads. This phenomenon creates the impression of differential responses of antibodies to desensitization. However, measuring the response of these same strong antibodies using titer values demonstrated predictable reduction.
Overall, these data highlight the importance of using dilutions to define antibody titers in determining candidacy, as well as a prognostic tool for planning and monitoring response to desensitization treatment.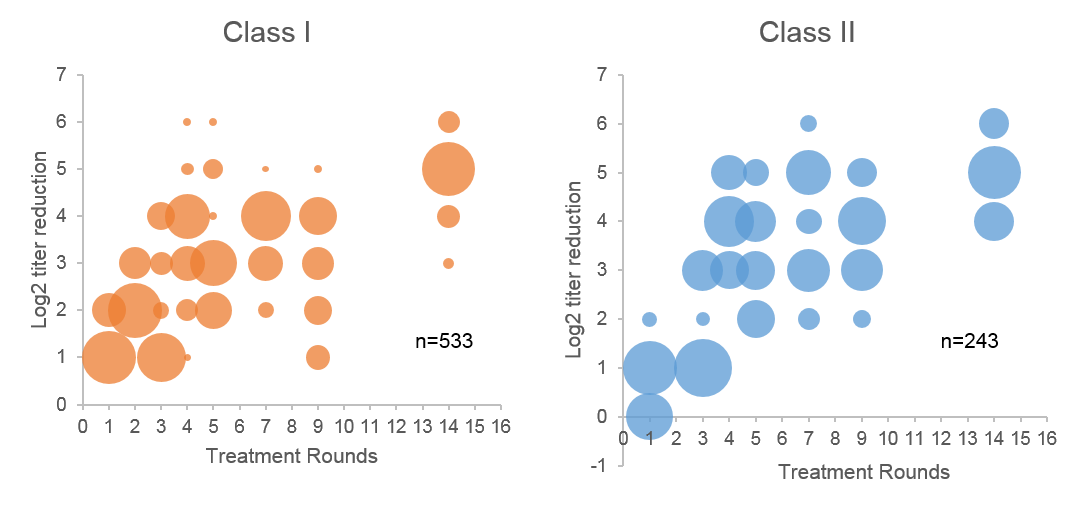
CITATION INFORMATION: Pinelli D, Zachary A, Friedewald J, Leffell M, Lucas D, Montgomery R, Tambur A. Prognostic Tools to Choose Candidates for Successful Desensitization. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Pinelli D, Zachary A, Friedewald J, Leffell M, Lucas D, Montgomery R, Tambur A. Prognostic Tools to Choose Candidates for Successful Desensitization. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/prognostic-tools-to-choose-candidates-for-successful-desensitization/. Accessed July 1, 2025.« Back to 2017 American Transplant Congress
