Primary Outcome of iWITH: A Multi-Center Clinical Trial of Complete Immunosuppression Withdrawal (ISW) in Stable Pediatric Liver Transplant (LT) Recipients.
1UCSF, San Francisco
2CCHMC, Cincinnati
3U Pitt, Pittsburgh
4Rho, Chapel Hill
5ITN, Bethesda
6U Mich, Ann Arbor
7NIAID/NIDDK/ITN, Bethesda.
Meeting: 2016 American Transplant Congress
Abstract number: 185.1
Keywords: Liver transplantation, Multicenter studies, Tolerance
Session Information
Session Name: Concurrent Session: Clinical Science: Tolerance: Clinical Studies
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:42pm-2:54pm
Presentation Time: 2:42pm-2:54pm
Location: Room 304
AIM: Test the hypothesis that a selected cohort of pediatric LT recipients can safely undergo complete ISW followed by assessment for operational tolerance (OT)
METHODS: 88 subjects [39(44%) male; 57(65%) deceased grafts] at 12 centers met clinical, biochemical, and histologic eligibility criteria (Fig). ISW occurred in 8 steps over 36-48 wks. ALT/GGT >100 IU/L mandated liver biopsy. OT, the 1[deg] endpoint, was defined per protocol by stable ALT, GGT and histology 1 yr after last IS dose, compared to study entry 2 yrs earlier. Associations between complete ISW and baseline clinical, immunologic (donor specific antibody) and histologic (including C4d score) factors were evaluated using logistic regression analyses.
RESULTS: 35/88 subjects failed ISW prior to OT assessment 2[deg] rejection [31 biopsy-proven (24/6/1 Banff mild/moderate/inadequate per central pathologist); 4 clinical]. No baseline clinical, immunologic or histologic factor predicted complete (n=53; 60%) vs. failed (n=35; 40%) ISW. Eight subjects are off IS for <1 yr and await OT assessment (last subject due in 3/2016). The remaining 45 subjects all met biochemical (ALT/GGT) OT criteria; 29 (64%) met histologic OT criteria [Fig]. Five subjects experienced 5 study-related serious adverse events: 3 related to liver biopsy (bile leak, cholangitis, cellulitis) and 2 related to ISW (in-patient rejection treatment with intravenous corticosteroids).
CONCLUSIONS: Selected pediatric LT recipients can safely attempt ISW. Notably, a significant proportion met biochemical but not histologic criteria for OT, suggesting a pivotal role for serial biopsies to assess for OT, detect clinically relevant graft changes and confirm long-term safety. Although baseline clinical, immunologic and histologic features did not predict ISW outcome, planned mechanistic assays may identify an OT biomarker and/or elucidate OT mechanisms.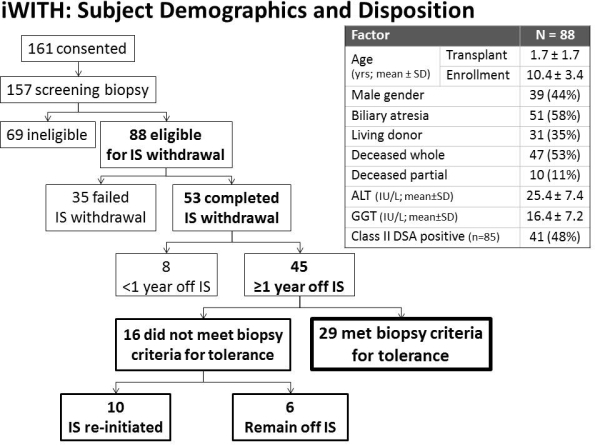
CITATION INFORMATION: Feng S, Bucuvalas J, Demetris A, Spain K, Kanaparthi S, Magee J, Mazariegos G, The iWITH Investigators Primary Outcome of iWITH: A Multi-Center Clinical Trial of Complete Immunosuppression Withdrawal (ISW) in Stable Pediatric Liver Transplant (LT) Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Feng S, Bucuvalas J, Demetris A, Spain K, Kanaparthi S, Magee J, Mazariegos G, Investigators TheiWITH. Primary Outcome of iWITH: A Multi-Center Clinical Trial of Complete Immunosuppression Withdrawal (ISW) in Stable Pediatric Liver Transplant (LT) Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/primary-outcome-of-iwith-a-multi-center-clinical-trial-of-complete-immunosuppression-withdrawal-isw-in-stable-pediatric-liver-transplant-lt-recipients/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
