Pig-To-Baboon Liver Xenotransplantation Utilizing Genetically Modified Swine
1Massachusetts General Hospital, Boston, MA, 2eGenesis, Cambridge, MA
Meeting: 2020 American Transplant Congress
Abstract number: A-279
Keywords: Liver transplantation, Primates, Survival, Xenoreactive antibodies
Session Information
Session Name: Poster Session A: Xenotransplantation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Pig-to-baboon liver xenotransplant (LXT) continues with significant hurdles, including thrombocytopenia, coagulation dysregulation and rejection with the longest reported survival of 29 days utilizing a GTKO pig. Herein, we report the results from six pig-to-baboon LXTs with novel genetically modified swine in an attempt to mitigate anti-xeno immunity and physiologic incompatibilities.
*Methods: 6 pig-to-baboon LXTs were performed, 2 each from 3 lines of triple knock out (TKO), CMV-negative genetically modified pigs (Pig 2.05, 2.09, 2.10) that varied in their expression of human transgenes for complement and coagulation pathway regulatory proteins, and genes targeting cellular immunity and inflammation. Immunosuppression consisted of anti-rhesus ATG, anti-CD20, corticosteroids, MMF and anti-CD154. All recipients received a continuous infusion of human Prothrombin Complex Concentrate. Graft function was assessed with standard laboratory analysis and graft histopathology.
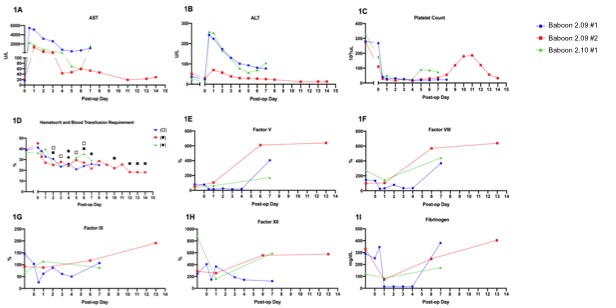
*Results: Two Pig 2.05 and one Pig 2.10 LTX recipients experienced pronounced reperfusion injury with uncontrollable hemorrhage and lung injury and were euthanized 6 hours post op. Of the 3 surviving animals, LFTs normalized between POD4-7. Each baboon manifest thrombocytopenia, with spontaneous recovery beginning on POD4 and 8. Consumption of coagulation factors occurred immediately after LXT, with subsequent production consistent with normal pig levels. Baboon 2.09#1 was euthanized on POD8 due to respiratory failure secondary to fluid overload; necropsy liver pathology showed focal ischemia without rejection and negative C4d. Baboon 2.09#2 had a normal protocol biopsy on POD8 but developed hemoptysis, necessitating euthanasia on POD14. Liver histology showed diffuse sinusoidal neutrophilic infiltrate, which may suggest an infectious process or xenograft rejection, however C4d was negative. Baboon 2.10#2 recovered uneventfully and required only one blood transfusion. On POD7, a rise in bilirubin and LFTs prompted exploration, where a bile leak with subsequent hepatic artery thrombosis was identified, requiring euthanasia. Liver histology confirmed focal subcapsular necrosis with negative C4d and no evidence of rejection.
*Conclusions: Our results of LXT with TKO pigs with modifications targeting complement, coagulation, immune and inflammation regulation demonstrate that antibody-mediated rejection-free survival is attainable without splenectomy or use of cobra venom factor.
To cite this abstract in AMA style:
Detelich D, Rickert CG, Coe TM, Kimura S, Rosales I, Deng H, Yeh H, Qin W, Kan Y, Layer J, Youd M, Westlin W, Yang L, Markmann JF. Pig-To-Baboon Liver Xenotransplantation Utilizing Genetically Modified Swine [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/pig-to-baboon-liver-xenotransplantation-utilizing-genetically-modified-swine/. Accessed February 23, 2026.« Back to 2020 American Transplant Congress