Peripheral Blood Immune Response-Related Gene Analysis for Evaluating the Development of Subclinical Antibody-Mediated Rejection
1Xenotransplantation, University of Alabama at Birmingham, Birmingham, AL
2Kidney Disease and Transplant Immunology, Aichi Medical University, Nagakute, Japan
3Biostatistics, Aichi Medical University, Nagakute, Japan
4Kidney Center, Nagoya Daini Red Cross Hospital, Nagoya, Japan
5Renal Transplant Surgery, Aichi Medical University, Nagakute, Japan.
Meeting: 2018 American Transplant Congress
Abstract number: A24
Keywords: Gene expression, HLA antibodies, Kidney transplantation
Session Information
Session Name: Poster Session A: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Background: Noninvasive methods for the early diagnosis of chronic antibody-mediated rejection (cAMR) are desired for patients with de novo (dn) donor-specific HLA antibody (DSA) because of the lack of effective methods other than graft biopsy. The aim of this study to elucidate the clinical relevance of immune-related gene expression in peripheral blood of kidney transplant recipients. Materials and Methods: Fourteen key molecules (Foxp3, CTLA-4, CCR7, TGF-β, IGLL-1, IL-10, ITCH, CBLB, Bcl-6, CXCR5, granzyme B, CIITA, Baff, TOAG-1/TCAIM) related to regulatory/cytotoxic function of immune cells were examined in the peripheral blood of 93 kidney transplant recipients by RT-PCR. The expression levels were compared among patients who had clinical cAMR with dn DSA (group A, N=16), subclinical cAMR with dn DSA (group B, N=17), negative cAMR with dn DSA (group C, N=21), and clinically stable function without dn DSA (group D, N=39). Results: On multivariate analysis, CIITA mRNA expression levels in groups B and C (potential risk groups for clinical cAMR) were significantly lower than those in group D (p < 0.01). Moreover, the CTLA-4 mRNA expression in group A (clinical cAMR group due to dn DSA) was significantly higher than that in groups B and C (p < 0.01). However, no biomarker could effectively differentiate between groups B and C. ROC curve analysis suggested that CIITA [area under the curve (AUC) = 0.902] and CTLA-4 (AUC = 0.785) may serve as valuable biomarkers of the stage of dn DSA production and clinical cAMR, respectively. Conclusions: In addition to dn DSA screening, monitoring of CIITA and CTLA-4 in peripheral blood could offer useful information on the time course of the development of cAMR. 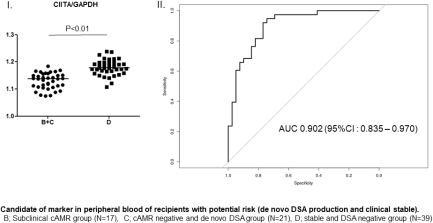
CITATION INFORMATION: Yamamoto T., Iwasaki K., Murotani K., Takeda A., Tsujita M., Hiramitsu T., Goto N., Narumi S., Watarai Y., Uchida K., Kobayashi T. Peripheral Blood Immune Response-Related Gene Analysis for Evaluating the Development of Subclinical Antibody-Mediated Rejection Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Yamamoto T, Iwasaki K, Murotani K, Takeda A, Tsujita M, Hiramitsu T, Goto N, Narumi S, Watarai Y, Uchida K, Kobayashi T. Peripheral Blood Immune Response-Related Gene Analysis for Evaluating the Development of Subclinical Antibody-Mediated Rejection [abstract]. https://atcmeetingabstracts.com/abstract/peripheral-blood-immune-response-related-gene-analysis-for-evaluating-the-development-of-subclinical-antibody-mediated-rejection/. Accessed March 9, 2026.« Back to 2018 American Transplant Congress
