p16INK4a Deletion in Vascular Endothelium Improves Kidney Regeneration Post Ischemia Reperfusion Injury
1Department of Kidney, Liver & Metabolic Diseases, MHH, Hannover, Germany
2Department of Nephrology, MHH, Hannover, Germany.
Meeting: 2018 American Transplant Congress
Abstract number: 297
Keywords: Endothelial cells, Graft function, Kidney transplantation, Renal injury
Session Information
Session Name: Concurrent Session: Endothelial Cell Biology
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 615/616/617
Background: Ischemia Reperfusion injury (IRI) mediated cellular senescence in kidney allografts has been linked to a loss in regenerative potential and delayed graft function. Injured renal vasculature plays a critical role in the pathophysiology of IRI where upregulation of p16INK4a induces endothelial dysfunction in the vascular endothelium limiting its capacity to form new vessels. This loss of microvascular reserve may exacerbate renal hypoxia further augmenting tubular injury and interstitial fibrosis. Further, the potential cross talk between senescent endothelium and renal epithelium in long term repair, tubular regeneration and development of chronic kidney disease (CKD) is not well investigated and constituted our main aim.
Methods: Mice with Tamoxifen inducible INK4a gene deletion in vascular endothelium (p16endo[Delta]flox/[Delta]flox) were generated to examine the effect of endothelial p16INK4a on renal regeneration post 30 days IRI. Kidneys were analysed for capillary rarefaction, tubular atrophy, fibrosis and immune infiltration by histology, immunostaining and RT-PCR.
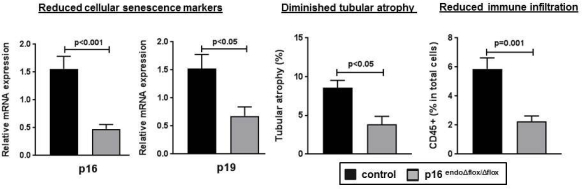
Results: The p16endo[Delta]flox/[Delta]flox kidneys displayed significantly reduced expression of pro-senescent cell cycle regulators p16INK4a and p19ARF. These kidneys also presented with significantly reduced chronic tubular damage, reduced expression of renal injury markers and diminished immune infiltration (Fig. 1). Although, p16endo[Delta]flox/[Delta]flox kidneys did not exhibit improved long-term microvascular density they did manifest improved endothelial function.
Conclusion: Our current results show that p16INK4a expression in endothelial cells contributes to maladaptive tubular epithelial repair and pro-inflammatory changes, while the selective ablation of vascular p16INK4a delays the onset of senescence and promotes renal regeneration. These observations are pivotal not only in better understanding of molecular mechanisms underlying acute kidney injury but designing targeted therapies to prevent its progression towards CKD.
CITATION INFORMATION: Melk A., Baisantry A., Wang S., Kubaink F., Bhayadia R., Schmitt R. p16INK4a Deletion in Vascular Endothelium Improves Kidney Regeneration Post Ischemia Reperfusion Injury Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Melk A, Baisantry A, Wang S, Kubaink F, Bhayadia R, Schmitt R. p16INK4a Deletion in Vascular Endothelium Improves Kidney Regeneration Post Ischemia Reperfusion Injury [abstract]. https://atcmeetingabstracts.com/abstract/p16ink4a-deletion-in-vascular-endothelium-improves-kidney-regeneration-post-ischemia-reperfusion-injury/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress