Outcomes of Induction Therapy with Bortezomib and High-Dose Intravenous Immunoglobulin for Kidney Transplantation with Preformed Donor-Specific Anti-Human Leukocyte Antigen Antibodies
Department of Urology, Ehime University, Toon, Japan
Meeting: 2019 American Transplant Congress
Abstract number: B209
Keywords: HLA antibodies, Induction therapy, Kidney transplantation, Sensitization
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Preformed donor-specific anti-human leukocyte antigen (HLA) antibodies (DSAs) are known to increase the risk of antibody-mediated rejection (ABMR) and graft failure. However, effective methods for avoiding poor prognosis have not been established for such cases. We aimed to test the outcomes of induction therapy with bortezomib and high-dose intravenous immunoglobulin (IVIG) for cases involving kidney transplantation with preformed DSA.
*Methods: We examined 64 living kidney transplant recipients from November 2013 to September 2018. Six recipients with preformed DSAs had positive reactions in crossmatch examinations. For these recipients, IVIG, at 2 to 4 g/kg, was administered during the perioperative period. Additionally, bortezomib, at 1.3 mg/m2, was administered on postoperative days 1, 4, 8, and 11. Rituximab was administered on postoperative day 1. On the day of bortezomib administration, plasma pheresis (PP) was performed (group A). Another eleven recipients with preformed DSA were administered rituximab and/or IVIG with/without PP (group B). Forty-seven recipient without preformed DSA were administered rituximab only in cases involving ABO incompatibility (group C).
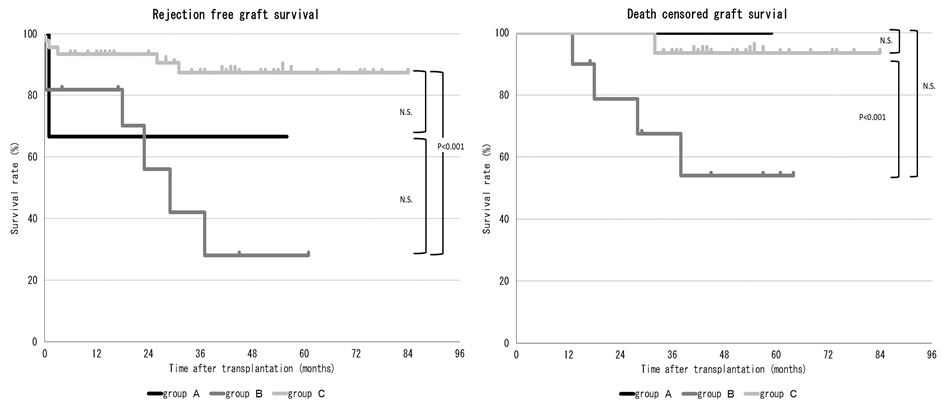
*Results: Rejection-free graft survival and death-censored graft survival were significantly lower in cases with preformed DSA than in those without preformed DSA (p<0.001 and p<0.05, respectively). The median median fluorescence intensity (MFI) level of class I and class II were 4937 and 13622 in group A, and 1503 and 2605 in group B, respectively. Both the MFI level of class I and class II were significantly higher in group A (p<0.001 for both). In group A, all recipients had positive reactions on crossmatch examinations (6/6, 100%) while in group B, only 2 patients had such positive reactions (2/11, 18%) (p<0.01). In comparison to group C, group B showed significantly lower rejection-free graft survival and death-censored graft survival; however, there was no significant difference in group A and C, although the recipients in group A had high immunological risk in comparison to those in group B (figure 1). The incidence of infectious diseases did not increase in group A in comparison to the other groups.
*Conclusions: Induction therapy with bortezomib and high-dose IVIG is useful in the short-term after kidney transplantation and improves treatment outcomes.
To cite this abstract in AMA style:
Miyauchi Y, Noda T, Miura N, Kikugawa T, Saika T. Outcomes of Induction Therapy with Bortezomib and High-Dose Intravenous Immunoglobulin for Kidney Transplantation with Preformed Donor-Specific Anti-Human Leukocyte Antigen Antibodies [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-induction-therapy-with-bortezomib-and-high-dose-intravenous-immunoglobulin-for-kidney-transplantation-with-preformed-donor-specific-anti-human-leukocyte-antigen-antibodies/. Accessed February 27, 2026.« Back to 2019 American Transplant Congress