Outcomes in Liver Transplantation with Use of Granulocyte-Colony Stimulating Factor
The Ohio State Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: 600
Keywords: Infection, Leukocytes, Liver transplantation, Neutropenia
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Questions remain if treatment with a granulocyte-colony stimulating factor (G-CSF) impacts clinical outcomes after liver transplant. The purpose of this study was to compare infection and rejection outcomes in liver transplant recipients with leukopenia that received intervention with G-CSF compared to those with no intervention(control).
*Methods: This retrospective single-center study evaluated adult liver transplant recipients transplanted between July 1, 2015 to June 29, 2018 who developed CTCAE grade 3 leukopenia (white blood cell (WBC) < 2 K/uL) during the first-year post-transplant. Patients were given G-CSF at physician discretion. Patients were excluded if they were diagnosed with graft versus host disease or died within 1-year post-transplant. The primary outcomes were the incidence of infection and rejection. Infections were defined as patients who required hospitalization and received antimicrobial treatment at the discretion of the physician during the first-year post-transplant. Patients were considered to have rejection if they had either biopsy proven acute rejection or elevated liver function tests that resolved with empiric steroid treatment. Secondary outcomes included time to and resolution of leukopenia.
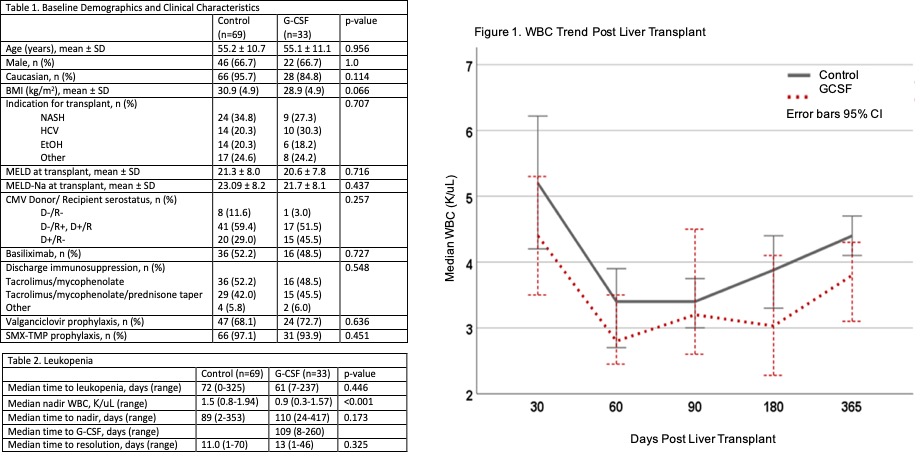
*Results: A total of 102 liver transplant recipients met inclusion criteria, 69 in the control and 33 in the G-CSF group. There was no difference in baseline characteristics (Table 1). After occurrence of leukopenia, approximately 40% of patients developed infection (Control: 39.7% (27/69) and G-CSF: 42.4% (14/33), p=0.794). Thirteen episodes of rejection occurred after development of leukopenia with no significant difference between the groups (Control: 10.1% (7/33) and G-CSF: 18.2% (6/69), p=0.255). The median time to onset of leukopenia was 72 days in the control group and 61 days in the G-CSF group (p=0.446) and leukopenia resolution occurred in 11 and 13 days (p=0.325) (Table 2). While the median WBC nadir was significantly different between the groups (Control: 1.5 K/uL and G-CSF: 0.9 K/uL (p<0.001), the overall median WBC trend for the first-year was similar (Figure 1).
*Conclusions: In the first year following liver transplantation, no difference was seen in the incidence of infection or rejection in liver transplant recipients with leukopenia that did or did not receive G-CSF intervention.
To cite this abstract in AMA style:
Schnelle K, Leino A, Chunduru M, Stein LVon, Witkowsky O. Outcomes in Liver Transplantation with Use of Granulocyte-Colony Stimulating Factor [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-in-liver-transplantation-with-use-of-granulocyte-colony-stimulating-factor/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress