Outcomes from De Novo Tacrolimus Weight-Based Dosing Compared to Conservative Dosing in Kidney Transplant Recipients.
1Vidant Medical Center/East Carolina University, Greenville, NC
2Duke University Hospital, Durham, NC.
Meeting: 2016 American Transplant Congress
Abstract number: D274
Keywords: FK506, Graft function, HLA antibodies, Kidney transplantation
Session Information
Session Name: Poster Session D: Poster Session II: Kidney Complications-Other
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Purpose: To compare de novo tacrolimus (TAC) weight based dosing (WDG) and conservative dosing (CDG) on incidence and duration of DGF in adult kidney transplant recipients.
Methods: This multicenter study compared 12 months of WDG (N=150, 0.05 mg/kg PO BID) to 12 months of historical CDG (N=161, 1 mg PO BID) from 2013-15. Exclusion criteria: patients with primary non-functioning grafts and those receiving drugs with TAC interactions. Secondary endpoints: time to therapeutic trough (8-10 ng/mL), eGFR, de novo donor specific HLA antibodies (DSA), and incidence of biopsy-proven acute rejection (BPAR) in first 90 days post-transplant.
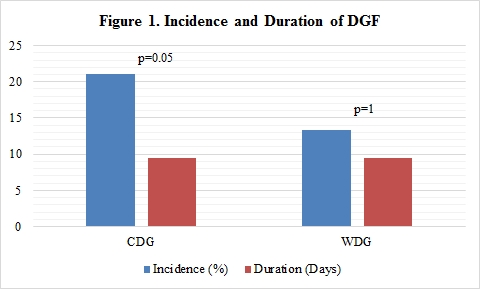
Results: Demographics were not statistically significant, except for HLA mismatch (4 WDG vs 5 CDG, p=0.04). Majority of recipients were AA (85 WDG vs 105 CDG) and had an average weight of 85 kg. KDPI, CIT, donor type, and pre-op duration of dialysis did not differentiate DGF outcomes. Increasing TAC dose from CDG to WDG did not adversely affect the overall duration of DGF as there was no difference between the two groups; however, incidence of DGF was statistically higher in CDG. Secondary endpoints were not significant with exception of mean time to therapeutic trough. First drawn TAC level >10 ng/mL was found in 22% of patients with DGF, 75% of those were in the WDG.
| Table 1. Secondary Outcomes | |||
| CDG (N=161) | WDG (N=150) | p-value | |
| Time to therapeutic trough, days | 15 (+24.7) | 6.6 (+5.8) | 0.0001 |
| eGFR (MDRD) at 90 days post-transplant, ml/min/1.73m2 | 52.9 (+16.9) | 51.9 (+19.9) | 0.49 |
| de novo DSA, n | 9 | 5 | 0.14 |
| BPAR, n Acute cellular rejection Antibody-mediated rejection Mixed |
14 9 2 3 |
7 6 0 1 |
0.18 |
Conclusion: WDG did not have a detrimental impact on duration of DGF or renal function in the first 90 days post-transplant with a significant decrease in time to therapeutic trough, and decreased incidence of de novo DSA and BPAR. Incidence of DGF was higher in the CDG, which may be due to ischemia/reperfusion injury unrelated to the TAC dose.
CITATION INFORMATION: Summers B, Szempruch K, Harris M, Quidley A, Haisch C, Maldonado A. Outcomes from De Novo Tacrolimus Weight-Based Dosing Compared to Conservative Dosing in Kidney Transplant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Summers B, Szempruch K, Harris M, Quidley A, Haisch C, Maldonado A. Outcomes from De Novo Tacrolimus Weight-Based Dosing Compared to Conservative Dosing in Kidney Transplant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-from-de-novo-tacrolimus-weight-based-dosing-compared-to-conservative-dosing-in-kidney-transplant-recipients/. Accessed July 12, 2025.« Back to 2016 American Transplant Congress
