Noninvasive Renal Transplant Graft Monitoring in Single Institution Using Cell-Free DNA in Recipient Plasma via Insertion-Deletion Allele Polymorphism.
1Urology, National University Hospital, Singapore, Singapore
2Laboratory Medicine, National University Hospital, Singapore, Singapore
3Nephrology, National University Hospital, Singapore, Singapore
Meeting: 2017 American Transplant Congress
Abstract number: D21
Keywords: Graft function, Kidney transplantation, Monitoring, Polymorphism
Session Information
Session Name: Poster Session D: Diagnostics/Biomarkers Session II
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Introduction
There is an unmet clinical need in renal transplantation towards early detection of donor organ damage. Serum creatinine(SCr) and biopsies are not ideal.
Cell-free donor DNA(cfdDNA) had been explored in cancer detection, prenatal diagnostics and solid organ transplants worldwide.
We aim to demonstrate ability in detection of cfdDNA in recipient plasma using real-time polymerase chain reaction of gender independent INDEL allele polymorphism and correlate the levels of cfdDNA with SCr at 1 year
Methods
10 Han Chinese donor-recipient pairs undergoing living related renal transplant were prospectively recruited from Aug 2014-Jun 2015. Immunosuppression regimen is per protocol. This is institution review board approved. Donor and recipient venous sampling performed, stored and analysed as per protocol.
Results
We demonstrate the ability to detect levels of cfdDNA in 9 of 10 recipient plasma post-kidney transplant. The changes in level of cfdDNA mirrored the levels of SCr immediately post-operative. 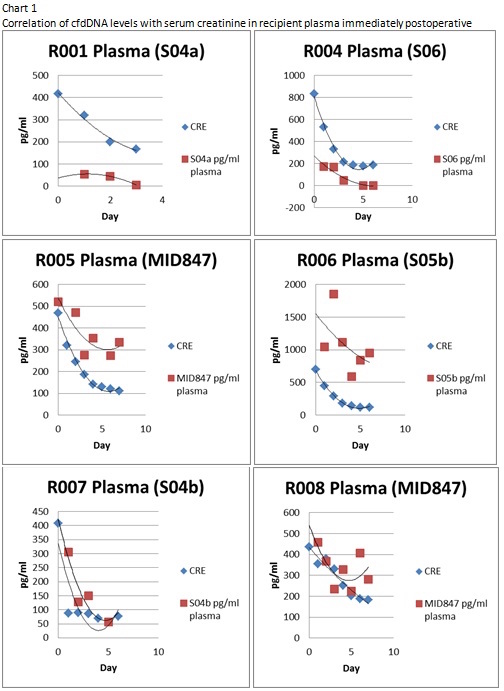 We correlated the levels of cfdDNA in recipient plasma on discharge with SCr and glomerular filtration rate at 1 year with good correlation(R2=0.81).
We correlated the levels of cfdDNA in recipient plasma on discharge with SCr and glomerular filtration rate at 1 year with good correlation(R2=0.81).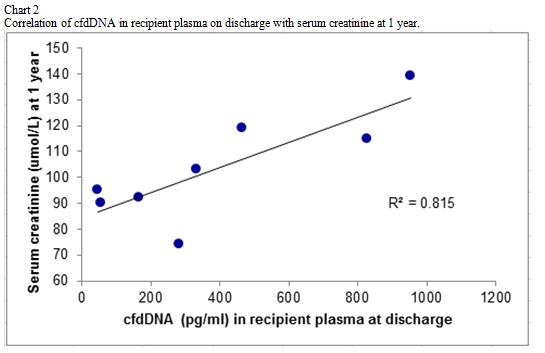 Conclusion
Conclusion
INDEL polymorphism is able to detect cfdDNA in recipient plasma post renal transplant.
cfdDNA has been shown to spike with insults to donor graft. Current results have achieved demonstrate ability of this novel technique to potentially monitor graft health via levels of cfdDNA in recipient plasma.
In spite of small numbers, this study suggests positive predictive value of stable cfdDNA levels for intermediate term renal function.
CITATION INFORMATION: Goh Y, Ho S, Raman L, Anantharaman V, Goh T, Koay S, Tiong H. Noninvasive Renal Transplant Graft Monitoring in Single Institution Using Cell-Free DNA in Recipient Plasma via Insertion-Deletion Allele Polymorphism. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Goh Y, Ho S, Raman L, Anantharaman V, Goh T, Koay S, Tiong H. Noninvasive Renal Transplant Graft Monitoring in Single Institution Using Cell-Free DNA in Recipient Plasma via Insertion-Deletion Allele Polymorphism. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/noninvasive-renal-transplant-graft-monitoring-in-single-institution-using-cell-free-dna-in-recipient-plasma-via-insertion-deletion-allele-polymorphism/. Accessed July 1, 2025.« Back to 2017 American Transplant Congress
