Next Gen ABO Antibody Assessment: Development of a Bead-Based ABO Antibody Detection Assay
1Dept of Pediatrics, Dept of Laboratory Medicine, GlycoNet, ATI, CDTRP, University of Alberta, Edmonton, AB, Canada, 2Dept of Pediatrics, ATI, GlycoNet, CDTRP, University of Alberta, Edmonton, AB, Canada, 3Dept of Laboratory Medicine, University of Alberta, Edmonton, AB, Canada, 4Dept of Chemistry, GlycoNet, CDTRP, University of Alberta, Edmonton, AB, Canada
Meeting: 2019 American Transplant Congress
Abstract number: A136
Keywords: Alloantibodies, Alloantigens, Immunoglobulins (Ig), Monitoring
Session Information
Session Name: Poster Session A: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
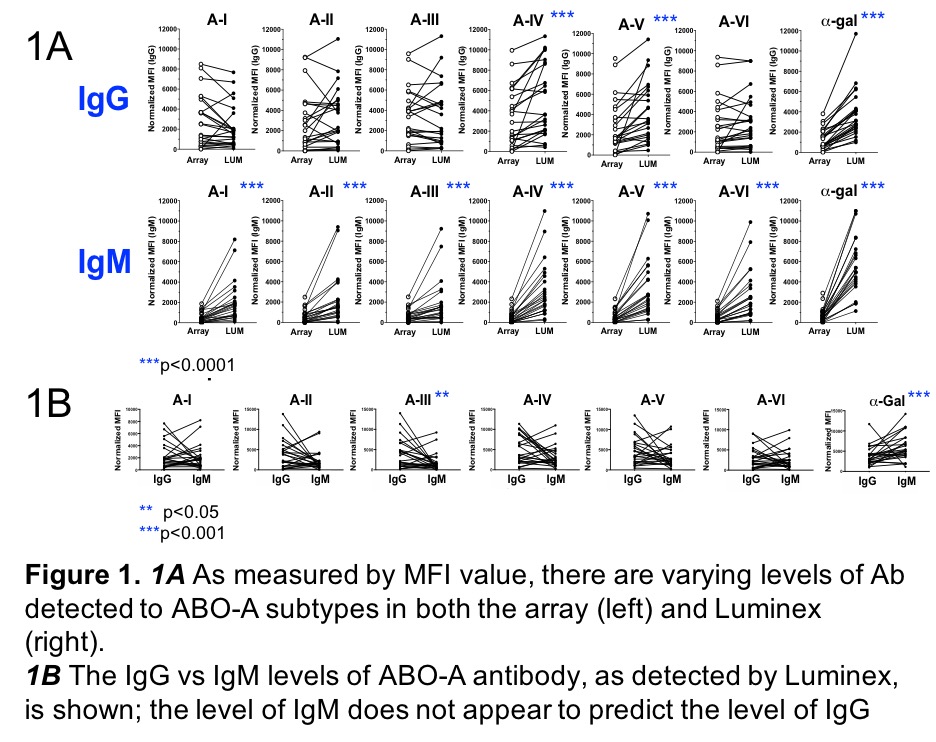
*Purpose: Accurate characterization of ABO antibodies (ABO-Ab) is critical to assess their impact in ABO incompatible (ABOi) transplantation. The current ABO-Ab detection method using erythrocyte agglutination is limited by lack of ABO-subtype specificity, difficulty in ABO-Ab isotype differentiation, and poor reproducibility. We previously developed an ABO glycan microarray method for ABO-Ab analysis to address these limitations. Our aim was to create a similar bead solid phase assay.
*Methods: ABO A-subtype antigens (I,II,III,IV,V,VI) were coupled to Luminex beads and quantified using monoclonal ABO-Ab. Bovine serum albumin and alpha-Gal antigen were coupled as negative/positive controls, respectively. IgG and IgM isotypes with specificities for ABO A-subtypes were measured and compared (n=39 healthy adult donors) by mean fluorescence intensity (MFI). These samples were tested in parallel on the glycan array. Wilcoxon signed-rank test was used.
*Results: ABO-A- subtype specific antibodies were detected; there was a high degree of variability in the MFI values between subjects. IgG and IgM ABO-A-Ab were detectable in all non-ABO A subjects although some subjects demonstrated low MFI values by both bead and array (Figure 1A). ABO-B controls had similar IgM ABO-A-Ab levels to ABO O controls but lower levels of IgG. Also shown in Fig 1A, IgM ABO-Ab were detected at higher MFI in the bead vs array method for many subtypes. IgG vs IgM antibodies were compared (Figure 1B); the level of antibody of one isotype does not appear to predict the level of the other.
*Conclusions: This method successfully measures ABO-A-Ab and shows promise for clinical laboratory implementation, where bead-based assays are already used. A flow cytometry bead panel is in development with similar potential. The specificity of this assay will facilitate assessment of ABO-Ab to subtypes, which are known to be expressed differently in cardiac endothelium than on erythrocytes. The ability to measure both IgM and IgG ABO antibodies makes it possible to evaluate of the role of each isotype in transplantation; isotype ABO-Ab differentiation may be particularly relevant in the setting of plasmapheresis, which more efficiently removes IgM antibodies. There is also potential for use in ABOi renal and stem cell transplantation.
To cite this abstract in AMA style:
Halpin A, Pearcey J, Motyka B, Maier S, Zhou J, Lowary TL, Cairo CW, West LJ. Next Gen ABO Antibody Assessment: Development of a Bead-Based ABO Antibody Detection Assay [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/next-gen-abo-antibody-assessment-development-of-a-bead-based-abo-antibody-detection-assay/. Accessed February 27, 2026.« Back to 2019 American Transplant Congress