Muscle-Liver Crosstalk via the Myokine MG53 Plays a Critical Role in Hepatocellular Repair
The Ohio State University Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: 192
Keywords: Ischemia, Liver, Liver transplantation
Session Information
Session Name: Basic: Ischemia Reperfusion & Organ Rehabilitation I
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
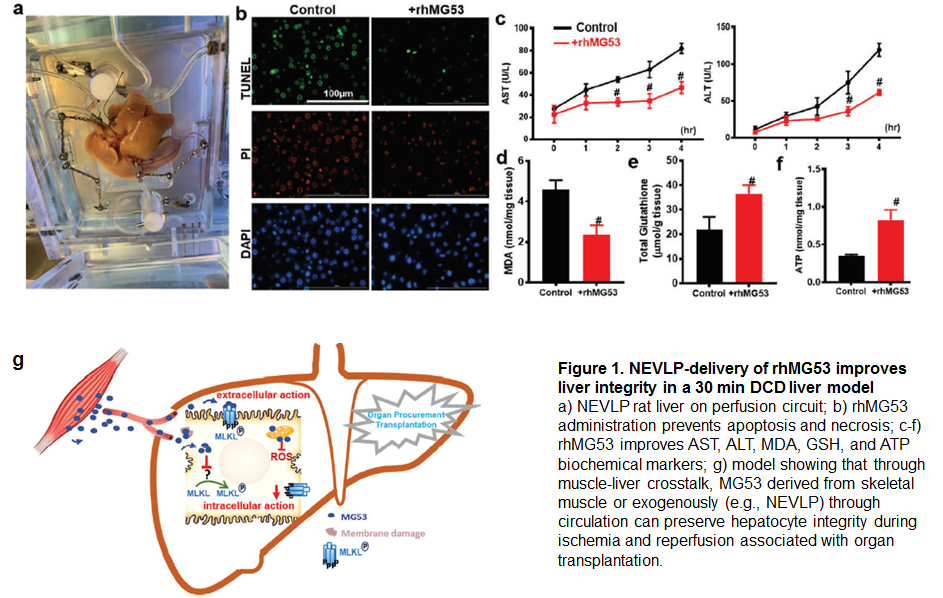
*Purpose: Transplantation remains the only effective treatment for end-stage liver disease, but the number of organs deemed suitable for transplantation falls far short of the demand. Donation after cardiac death (DCD) is a potential underutilized pool of donor livers. However, prolonged warm ischemia and subsequent reperfusion injury (IRI) increases the chances of organ dysfunction or non-function following transplantation. MG53 is a TRIM protein primarily expressed in muscle and has been identified to play a critical role in cell membrane repair. While the liver contains no endogenous MG53, we present data to support that muscle-liver crosstalk via MG53 as a myokine is integral in facilitating repair of hepatocellular injury.
*Methods: In vitro studies used primary rat and mouse liver cells and HEPG2 cells treated with recombinant human (rhMG53), subjected to hypoxia (1.1% O2) and reoxygenation injury and assessed by live cell imaging and measures of cell viability. In vivo, mice were subjected to a segmental hilar clamp model to induce IRI. Ex vivo, rat livers were subjected to normothermic ex-vivo liver perfusion (NEVLP) with or without rhMG53. For all in vivo and ex vivo studies, liver injury markers and histology were assessed.
*Results: Biochemical and live cell imaging studies reveal that, during injury, MG53 translocates from circulation to target injured hepatocytes. Additionally, endogenous MG53 as a myokine promotes hepatocellular repair by membrane-delimited interaction with MLKL (a pore forming protein) to reduce pore-forming activity and to preserve hepatocellular integrity. In vitro, in vivo, and ex vivo exogenous rhMG53 administration during injurious conditions results in improvement in cell integrity and mitigation of IRI through upregulation of cell survival signaling.
*Conclusions: Our findings show a novel pathway for MG53 in liver repair with both extracellular and intracellular actions and define the physiological role of muscle-derived MG53 in hepatoprotection. Exogenous administration of rhMG53 significantly mitigates hepatic IRI, thus establishing a therapeutic target for mitigating hepatic IRI as seen during transplantation. In all, rhMG53 may allow for the expansion of the donor organ pool along with a subsequent improvement in liver transplant outcomes.
To cite this abstract in AMA style:
Akateh C, Ko J, Reader BF, Kim J, Lee Y, Whitson BA, Ma J, Black SM. Muscle-Liver Crosstalk via the Myokine MG53 Plays a Critical Role in Hepatocellular Repair [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/muscle-liver-crosstalk-via-the-myokine-mg53-plays-a-critical-role-in-hepatocellular-repair/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress