Integrated Magnetic-Electrochemical Exosome (iMEX) Technology to Detect Rejection in Kidney Transplant Recipients.
Transplantation Research Center, Harvard Medical School, Boston
Exosome Diagnostic Inc, Cambridge
Meeting: 2017 American Transplant Congress
Abstract number: D118
Keywords: Biopsy, CD3, Rejection, T cell graft infiltration
Session Information
Session Name: Poster Session D: Kidney: Acute Cellular Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Methods for monitoring clinical rejection including increases in serum creatinine and urinary protein excretion may not reflect subclinical rejection. Similarly repeat biopsy may result in increased procedural complications and cost. An accurate non-invasive method would allow for earlier diagnosis and minimize the amount of immunosuppression needed for adequate treatment. Extracellular vesicles such as exosomes are released by cells and carry the parent cells' surface proteins and nucleic acids. Vesicles can be isolated and their surface protein profile probed. In the transplanted kidney, exosomes will stem from podocytes, renal tubular cells and from immune cells, generated during rejection.
After initial screening of multiple surface proteins, we show that human T cells secrete exosomes that express surface CD3. Detecting those CD3+ exosomes in the urine can potentially diagnose cellular rejection. While, we recently described a novel integrated magnetic-electrochemical exosome (iMEX) technology that integrates both vesicle isolation and detection into a single platform, we detected CD3+ exosome isolated from supernatants of activated human T cells in vitro.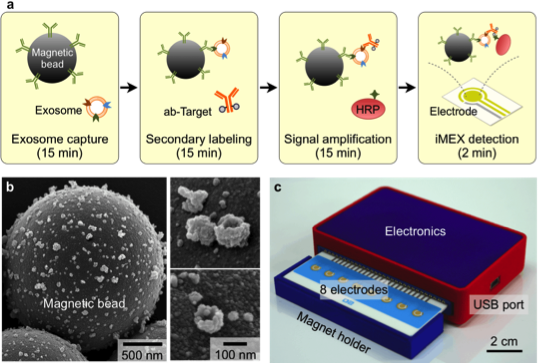 We then applied iMEX to urine samples collected from patients undergoing a transplant kidney biopsy for clinical indications. The initial discovery study on urine samples of 20 patients showed a strong correlation between higher level of CD3+ exosomes and patients undergoing cellular rejection, with AUC >0.9.
We then applied iMEX to urine samples collected from patients undergoing a transplant kidney biopsy for clinical indications. The initial discovery study on urine samples of 20 patients showed a strong correlation between higher level of CD3+ exosomes and patients undergoing cellular rejection, with AUC >0.9.
Furthermore, a validation study on a larger independent cohort showed similar correlation with AUC >0.8. 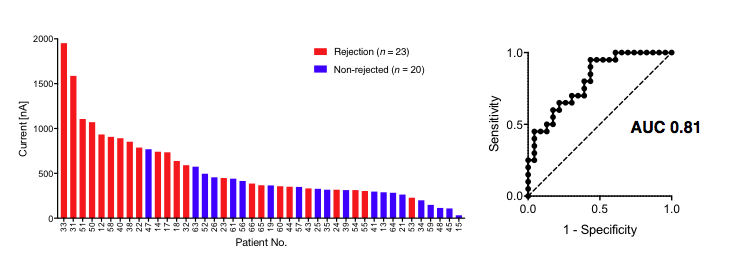 The aim of this research is to develop a new, fast, cost-effective iMEX platform for early rejection detection, improving patient outcome and reducing complications.
The aim of this research is to develop a new, fast, cost-effective iMEX platform for early rejection detection, improving patient outcome and reducing complications.
CITATION INFORMATION: Assaker J, Park J, Routray S, Eskandari S, Lee H, Azzi J. Integrated Magnetic-Electrochemical Exosome (iMEX) Technology to Detect Rejection in Kidney Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Assaker J, Park J, Routray S, Eskandari S, Lee H, Azzi J. Integrated Magnetic-Electrochemical Exosome (iMEX) Technology to Detect Rejection in Kidney Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/integrated-magnetic-electrochemical-exosome-imex-technology-to-detect-rejection-in-kidney-transplant-recipients/. Accessed July 12, 2025.« Back to 2017 American Transplant Congress
