In-Vivo Application of Ides (Imlifidase) in Nonhuman Primates Results in Xenoreactive Desensitization: A Novel Anti-Humoral Therapy
1Emory University School of Medicine, Atlanta, GA, 2University of Miami, Miami, FL, 3HANSA Biopharma, Lund, Sweden
Meeting: 2020 American Transplant Congress
Abstract number: 102
Keywords: Antibodies, Highly-sensitized, Immunoglobulins (Ig), Xenoreactive antibodies
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:27pm-4:39pm
Presentation Time: 4:27pm-4:39pm
Location: Virtual
*Purpose: Antibody-mediated rejection is a formidable barrier to xenograft survival. A novel anti-humoral IdeS therapy, which cleaves serum IgG, represents a promising therapy to reduce circulating antibody against porcine xenografts.
*Methods: In-vitro: Highly xenosensitized rhesus macaques with high anti-pig antibody titers (N=2) were selected. A flow cytometry-based xenoreactive crossmatch assay was performed in the presence of varying concentrations of IdeS. In-vivo: Rhesus macaques with moderate-high pre-treatment xenoreactive antibody titers (N=3) were selected. Each received a single dose of IdeS 10mg/kg IV followed by serial serum sampling, which was analyzed via flow cytometry-based xenoreactive crossmatch assays.
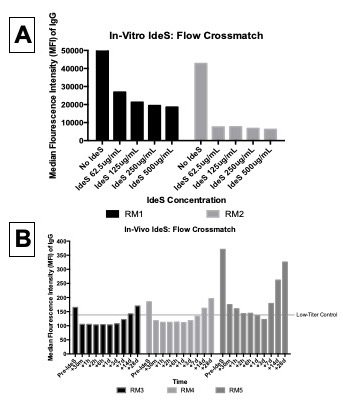
*Results: In-vitro application of IdeS results in reduction of xenoreactive antibody (Panel A) among highly sensitized rhesus macaque serum. A dose-dependent effect was observed with higher concentrations of IdeS resulting in more complete xenoreactive IgG depletion. This reduction in xenoreactive IgG was associated with a concomitant reduction in complement-dependent cytotoxicity (data not shown) to the level of non-exposed pig cells. . In-vivo administration of Ides to moderate-titer rhesus macaques resulted in rapid depletion of xenoreactive IgG, within 30 minutes of dosing (Panel B). This effect was sustained for approximately 14 days, when xenoreactive titers returned to their pre-IdeS baseline. All three monkeys, regardless of pre-IdeS titer, demonstrated a reduction in xenoreactive DSA to the level of a low-titer control recipient. There were no adverse events associated with IdeS administration.
*Conclusions: IdeS can successfully reduce xenoreactive antibody binding and complement-dependent cytotoxicity. Both in-vitro and in-vivo IdeS administration demonstrates a reduction in IgG binding and complement-dependent cytotoxicity, with effects within 30 minutes of IdeS treatment and lasting up to 14 days. In-vivo administration of IdeS demonstrates substantial effect in xenoreactive IgG reduction, representing a promising target for xenotransplant applications, including the ability to transiently desensitize a highly xenoreactive NHP recipient as part of a pretransplant induction strategy or as rescue therapy for IgG-mediated xenograft rejection.
To cite this abstract in AMA style:
Lovasik BP, Matar AJ, Faber DA, Mathews DV, Breeden C, Kim SC, Tector AJ, Robertson A, Kjellman C, Adams AB. In-Vivo Application of Ides (Imlifidase) in Nonhuman Primates Results in Xenoreactive Desensitization: A Novel Anti-Humoral Therapy [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/in-vivo-application-of-ides-imlifidase-in-nonhuman-primates-results-in-xenoreactive-desensitization-a-novel-anti-humoral-therapy/. Accessed February 24, 2026.« Back to 2020 American Transplant Congress