In a Murine Model of Acetaminophen-Mediated Liver Injury, Tubastatin-a, An Hdac6-Specific Inhibitor, Improves Survival and Decreases Hepatocellular Injury
1Department of Surgery, University of Pennsylvania, Philadelphia, PA, 2Department of Pathology, Children's Hospital of Pennsylvania, Philadelphia, PA, 3Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: 408
Keywords: Hepatocytes, Liver failure, Liver transplantation, Tissue-specific
Session Information
Session Name: Basic: Ischemia Reperfusion & Organ Rehabilitation II
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Tylenol (APAP) toxicity, due to its narrow therapeutic window, is the leading cause of acute liver failure in the United States, resulting in approximately 30,000 hospital admissions and 500 deaths annually in spite of the use of N-acetyl-cysteine (NAC) therapy. Histone deacetylases (HDACs) are enzymes that alter gene expression, regulating diverse cellular processes. We have previously shown that Tubastatin-A (TubA), an HDAC6 inhibitor, provides protection from hepatic ischemia reperfusion injury. We now report that TubA is also protective and increases survival in a murine model of acetaminophen toxicity.
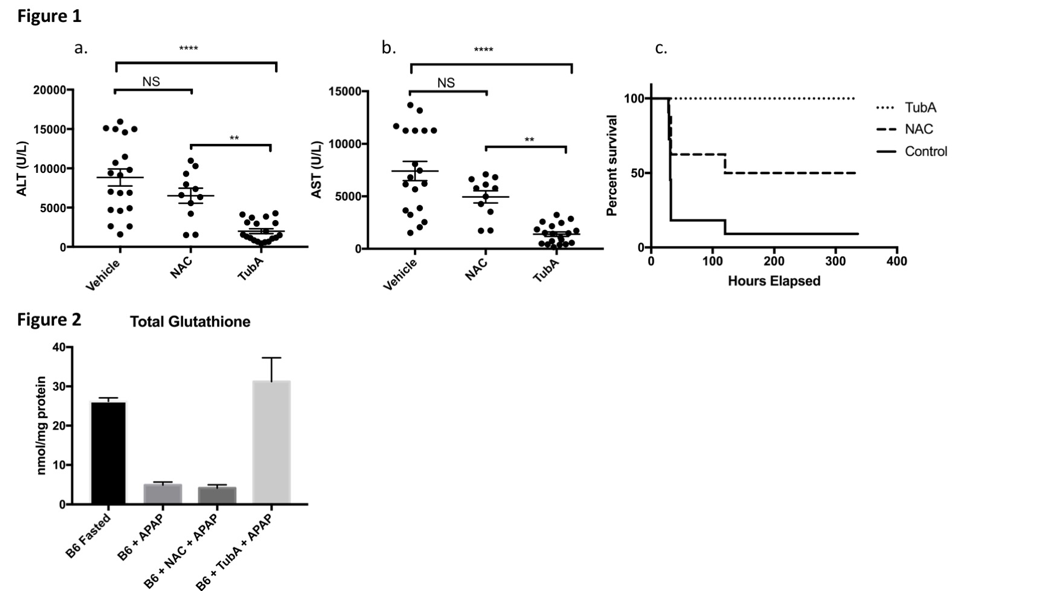
*Methods: Fasted, male wild type C57BL/6 (B6) mice were treated with vehicle, TubA, or NAC, at 16 hours and one hour prior to administration of APAP (500 mg/kg). Serum ALT and AST were measured in each group 24 hours after APAP administration. Each group was monitored for 14 days by an investigator blinded to treatment condition; time of death was recorded. Liver glutathione was measured in fasted B6 mice, and in mice treated with either vehicle, NAC, or TubA 6 hours after APAP induced liver injury.
*Results: TubA-treated mice developed significantly less hepatocellular injury compared to controls or mice treated with NAC after APAP administration (Fig 1a/b). TubA-treated mice had improved survival compared to controls or NAC-treated mice (Fig 1c). Liver glutathione levels are significantly reduced after APAP administration in controls and NAC-treated mice but are preserved in TubA-treated mice (Figure 2).
*Conclusions: TubA provides greater protection than NAC in an APAP model of liver injury. TubA mediated protection against APAP induced liver injury is associated with increased hepatic glutathione stores. These new findings, taken together with previously shown effects in hepatic ischemia reperfusion injury, suggest that TubA is a potent inhibitor of hepatocellular injury.
To cite this abstract in AMA style:
OBrien CS, Hernandez PT, Concors SJ, Krumeich LN, Wang Z, Ge G, Bhatti T, Kozikowski A, Hancock W, Levine MH. In a Murine Model of Acetaminophen-Mediated Liver Injury, Tubastatin-a, An Hdac6-Specific Inhibitor, Improves Survival and Decreases Hepatocellular Injury [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/in-a-murine-model-of-acetaminophen-mediated-liver-injury-tubastatin-a-an-hdac6-specific-inhibitor-improves-survival-and-decreases-hepatocellular-injury/. Accessed February 24, 2026.« Back to 2020 American Transplant Congress