Impact of Varying Levels of Normal Range Baseline Donor Derived Cell Free DNA on Medium-Term Outcomes Following Kidney Transplantation
1Nephrology, Medicine Institute, Allegheny General Hospital, Pittsburgh, PA, 2Abdominal Transplantation, Allegheny General Hospital, Pittsburgh, PA
Meeting: 2022 American Transplant Congress
Abstract number: 1563
Keywords: Graft function, Proteinuria, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Donor derived cell free DNA (dd-cfDNA) is a biomarker with high negative predictive value for ruling out acute rejection (AR) in kidney allografts. Baseline dd-cfDNA levels are <1% in 96% of kidney transplant recipients (KTRs) and is generally considered as normal. Incremental dd-cfDNA levels within "normal range" could potentially be reflective of varying levels of allograft health. We tested whether low-normal vs. high-normal baseline dd-cfDNA values in the early post- transplant period would have differing impact on medium-term outcomes including allograft function, AR and proteinuria in KTRs.
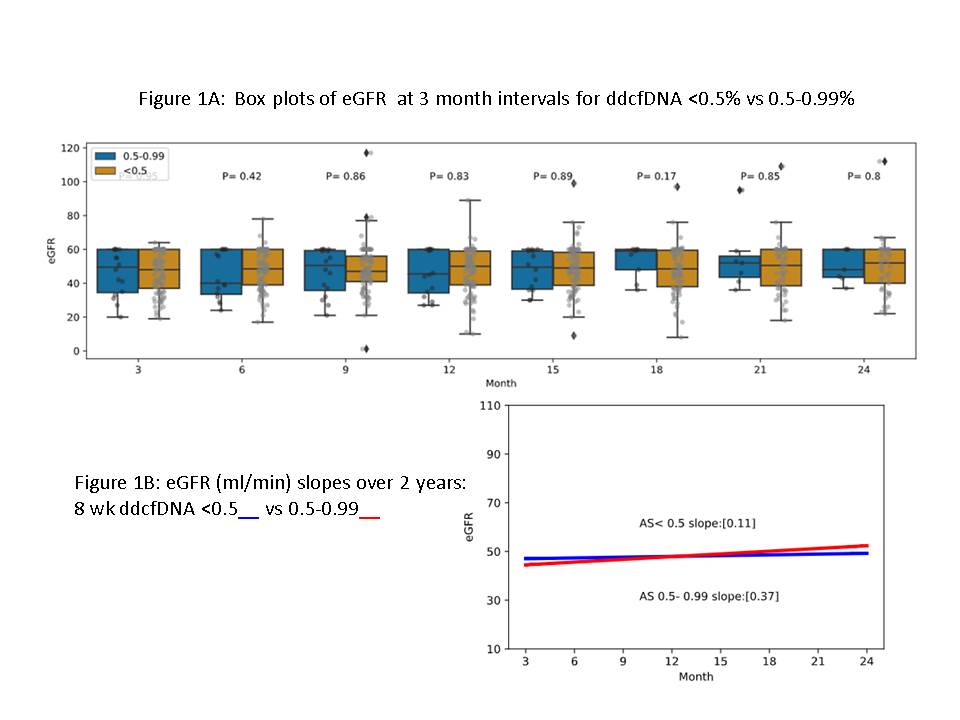
*Methods: KTRs who underwent transplantation at our center between September 2017 and June 2020 who had dd-cfDNA (AlloSure, CareDx, Brisbane, CA) levels done at or around 8 weeks post-transplantation with levels <1.0% were included in the analysis. These patients were divided into 2 groups: group 1 with dd-cfDNA <0.5% (low-normal) and group 2 with dd-cfDNA 0.5-0.99% (high-normal). Estimated glomerular filtration rates (eGFR) between the groups at 3 month intervals were compared using box plots and longitudinal eGFR up to 2 years post-transplant were compared using linear mixed model. Incidence of biopsy proven AR within 12 months as well as any degree of dipstick proteinuria at 12 months of transplantation were compared between the 2 groups.
*Results: There were 111 patients included in the analysis including 62 males. Among the study group, 39 had living and 72 received deceased donor kidneys. There were 95 patients in group 1, and 16 patients in group 2. There were no differences observed in 3-month interval cross-sectional eGFRs (fig 1A) or 2 -year longitudinal eGFRs (fig 1B) between the groups. Of the 95 patients in group one, 13 (13.7%) developed AR whereas no patient in group 2 (n=16) developed AR within first 12 months (incidence rate [95% CI]: 0.14 [0.07-0.24] vs. 0 [0-0.23], p=0.14). Proteinuria was detected at 12 months in 61 (64%) patients in group 1 and 8 (50%) patients in group 2 (incidence rate [95% CI]: 0.64 [0.49-0.82] vs. 0.50 [0.22-0.99], p=0.52).
*Conclusions: Our analysis found no differences between early post-transplant low-normal and high-normal baseline dd-cfDNA levels in terms of outcomes including eGFR up to 2 post-transplant years as well as incidence of AR within and proteinuria at 12 months of kidney transplantation. These findings support the commonly practiced use of 1% cut off as a threshold to separate normal from abnormal dd-cfDNA levels.
To cite this abstract in AMA style:
Sureshkumar KK, Grazier A, Chopra B. Impact of Varying Levels of Normal Range Baseline Donor Derived Cell Free DNA on Medium-Term Outcomes Following Kidney Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-varying-levels-of-normal-range-baseline-donor-derived-cell-free-dna-on-medium-term-outcomes-following-kidney-transplantation/. Accessed July 15, 2025.« Back to 2022 American Transplant Congress