Impact of Dysgeusia on Transplant Recipients with Refractory Cytomegalovirus with/without Resistance Receiving Maribavir: Post-Hoc Analyses from a Phase 3 Randomized Trial
F. Silveira1, S. Haider2, D. Kaul3, O. Witzke4, J. Gu5, A. Sundberg5
1Univ. Pittsburgh, Pittsburgh, PA, 2McMaster Univ., Hamilton, ON, Canada, 3Univ. Michigan, Ann Arbor, MI, 4Univ. Duisburg-Essen, Essen, Germany, 5Takeda Development Center Americas, Inc., Lexington, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1346
Keywords: Adverse effects, Cytomeglovirus, Safety, Viral therapy
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) III
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Hall C
*Purpose: In a Phase 3 study (SOLSTICE; NCT02931539), maribavir (MBV) was superior to investigator-assigned therapies (IAT; val/ganciclovir, foscarnet, or cidofovir) for CMV clearance at Wk 8 in transplant recipients with refractory CMV with/without resistance. Dysgeusia, a taste-disturbance disorder, was the most common treatment-emergent adverse event (TEAE) in the MBV arm. We report the impact of dysgeusia on patients (pts) treated with MBV in SOLSTICE.
*Methods: Pts were randomized 2:1 to 400 mg MBV BID or IAT for 8 wks tx and 12-wk follow-up. After ≥3 wks tx, pts on IAT could enter a MBV rescue arm if they met pre-specified criteria. Primary endpoint: time to confirmed CMV clearance at Wk 8. TEAEs were evaluated. Dysgeusia (ageusia, dysgeusia, hypogeusia, and/or taste disorder) was an AE of special interest. Post-hoc analyses included assessment of primary endpoint and change in body weight in pts with/without dysgeusia.
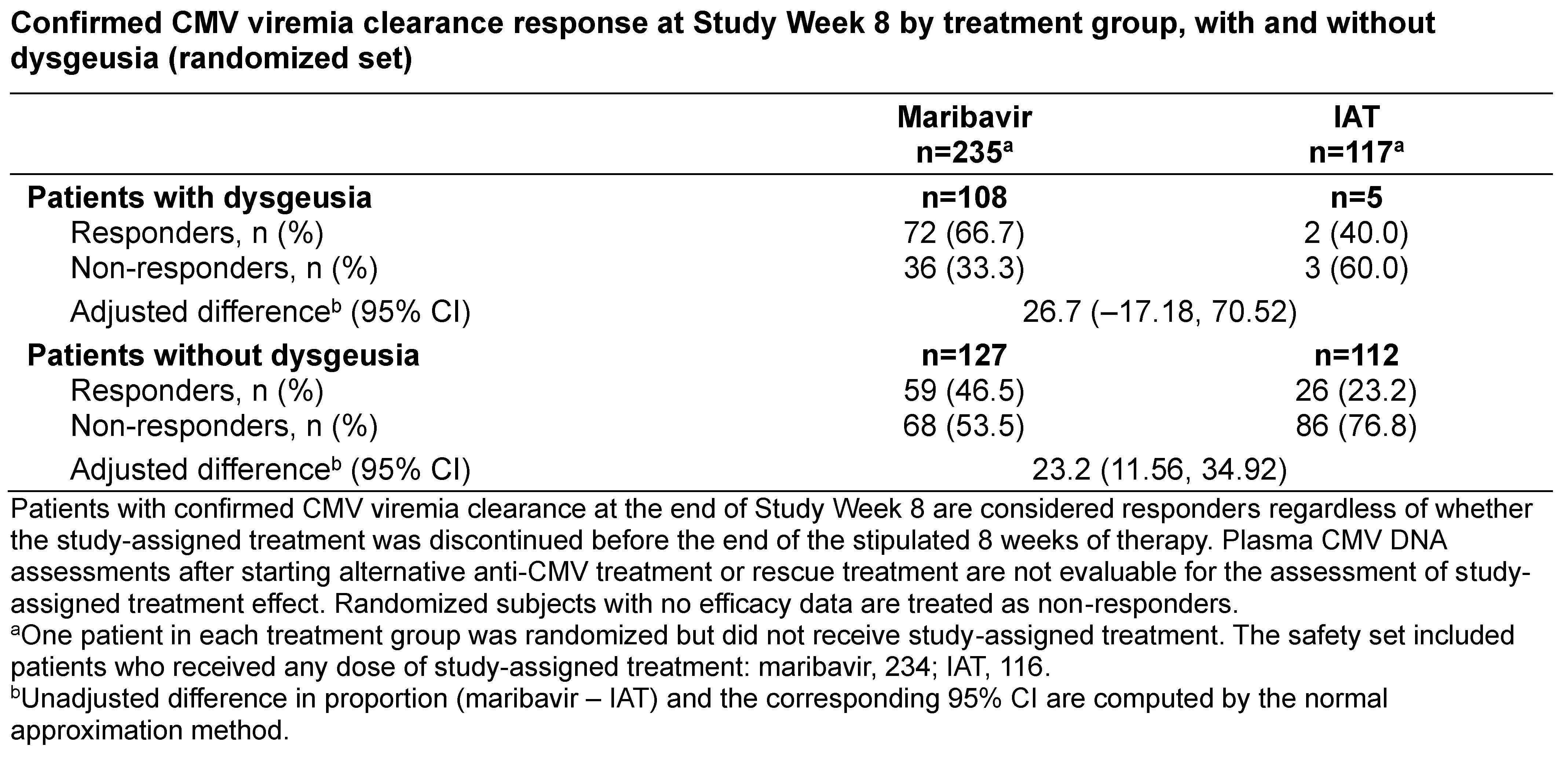
*Results: Overall, 352 pts were randomized; 234 received study-assigned MBV, of whom 108 (46.2%) had dysgeusia during the on-tx period; considered tx-related in 103 (44.0%). Dysgeusia was mild (n=86) or moderate (n=17) in severity for pts in the MBV arm (missing, n=5). Only 2 (0.9%) pts on MBV discontinued tx due to dysgeusia. The primary endpoint was achieved in 72 (66.7%) pts on MBV and 2 (40.0%) pts on IAT who had dysgeusia, and 59 (46.5%) and 26 (23.2%) pts without dysgeusia, respectively (Table). Median change from baseline (min, max) in body weight (kg) at Wk 8 was similar in pts on MBV with and without dysgeusia (0.9 [−9, 9] vs 0.1 [−16, 16]), and in pts with dysgeusia in MBV and IAT (0.9 [−9, 9] vs −0.9 [−9, 8]). For pts who had MBV as study-assigned or rescue tx (n=256), median tx duration (days [range]) was 57 (2-64) (study-assigned) and 57 (22-60) (rescue). In pts reporting dysgeusia (study-assigned, n=108; rescue, n=11) resolution was seen on-tx in 44 (37%) pts; ongoing dysgeusia at last tx date (n=75, 63%) resolved off-tx in 67 (89.3%) pts.
*Conclusions: In SOLSTICE, dysgeusia was common in the MBV arm but was generally mild/moderate. In these post-hoc analyses in pts with/without dysgeusia, the proportion of pts who achieved CMV clearance at Wk 8 was higher in MBV than IAT reflecting the SOLSTICE primary endpoint analysis. Dysgeusia had almost no effect on body weight during the on-tx study period. Funding: Takeda Development Centre Americas, Inc.
To cite this abstract in AMA style:
Silveira F, Haider S, Kaul D, Witzke O, Gu J, Sundberg A. Impact of Dysgeusia on Transplant Recipients with Refractory Cytomegalovirus with/without Resistance Receiving Maribavir: Post-Hoc Analyses from a Phase 3 Randomized Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-dysgeusia-on-transplant-recipients-with-refractory-cytomegalovirus-with-without-resistance-receiving-maribavir-post-hoc-analyses-from-a-phase-3-randomized-trial/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress