Impact of Catch-Up Vaccinations on Anti-HLA Antibody Responses in Pediatric Kidney Transplant Candidates
1University Health System, San Antonio, The University of Texas Health Science Center at San Antonio, San Antonio, TX, 2Loyola University Medical Center, Chicago, IL, 3University Health System, San Antonio, TX, 4The University of Texas Health Science Center at San Antonio, San Antonio, TX, 5University of Colorado Health, Aurora, CO
Meeting: 2020 American Transplant Congress
Abstract number: A-065
Keywords: Histocompatibility, HLA antibodies, HLA antigens, Immunogenicity
Session Information
Session Name: Poster Session A: Kidney: Pediatrics
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Evaluate antibody (Ab) response after catch-up vaccinations in pediatric kidney transplant candidates (PKTC).
*Methods: A single center retrospective review of PKTC less than or equal to 18 years old who received catch-up vaccinations from 2/2017 to 11/2019 was conducted. Anti-HLA Ab were detected at 3-4 and 7-10 weeks post catch-up vaccinations by single antigen assay. De novo anti-HLA Ab was defined as a change from negative mean fluorescence intensity (MFI) to positive MFI. Positive MFI strength categories include weak: 1000 – 2999; moderate: 3000 – 9999; strong: greater than 10,000.
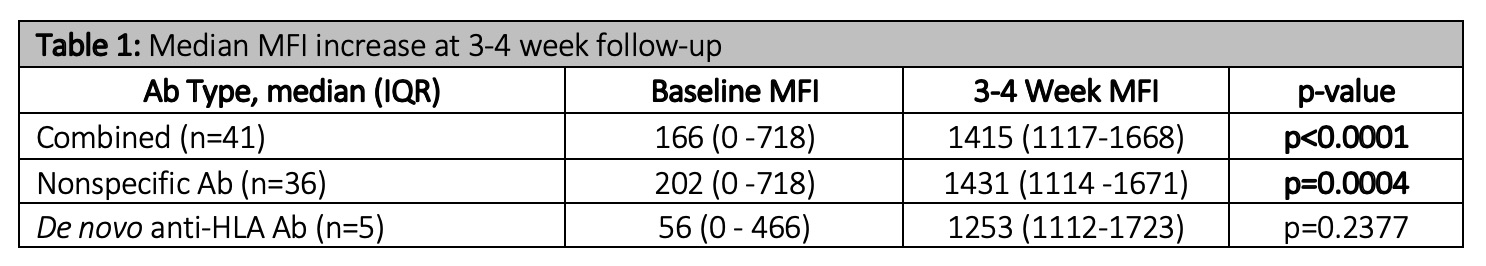
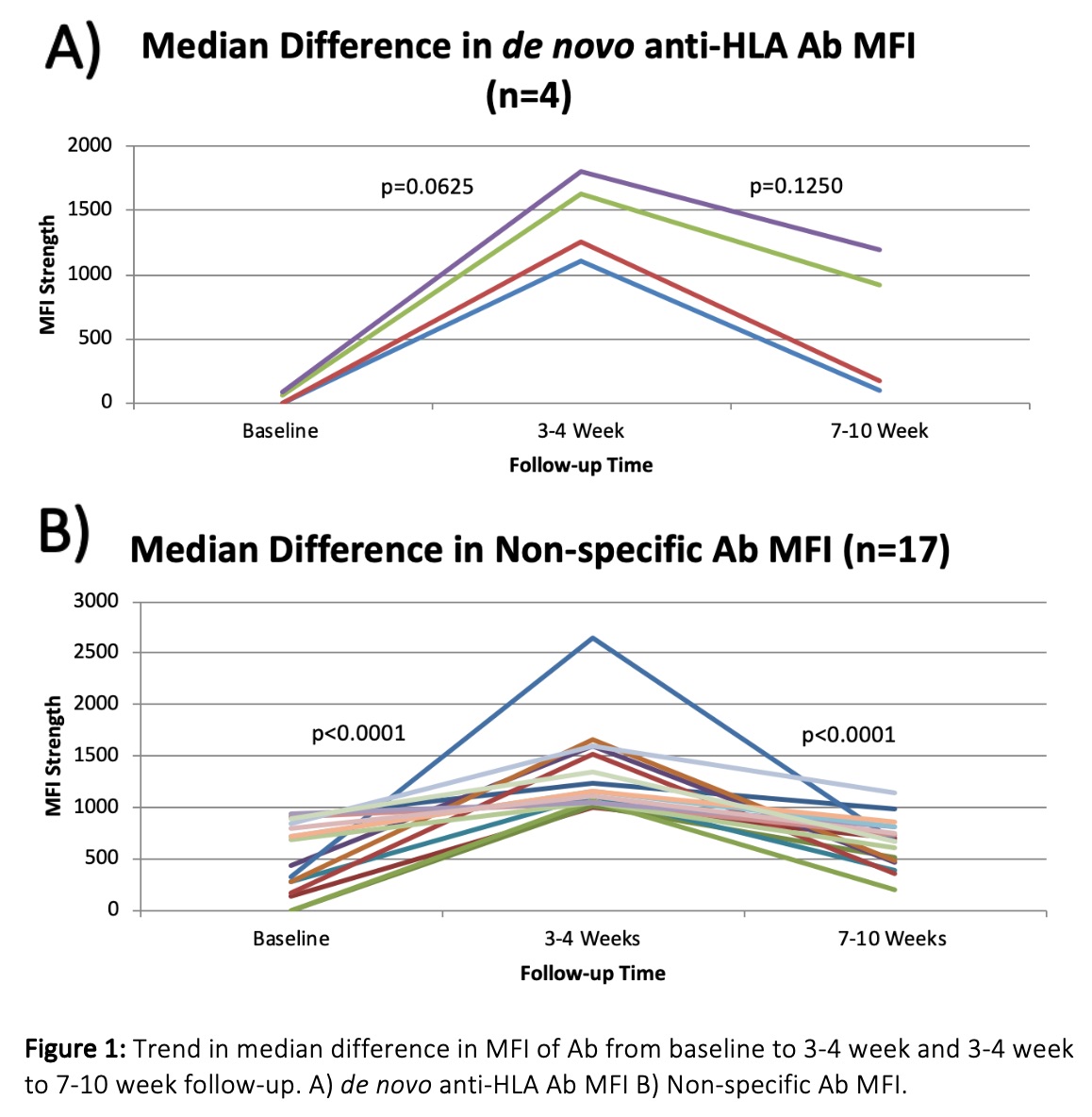
*Results: Sixty-four vaccinations were administered to 14 patients. Forty-one potential de novo anti-HLA Ab were detected in 5 patients, all without history of sensitizing events from time of evaluation to follow-up. After additional Ab panel testing, 5/41 potential de novo anti-HLA Ab were determined to be HLA specific; the remaining 36 were deemed non-specific. Median MFI increase was significant for non-specific, but not de novo anti-HLA Ab (Table 1). Median MFI values peaked at 3-4 week but diminished by 7-10 week follow-up (Figure 1). No HLA-donor specific Ab developed from the time of transplant to the end of follow-up in the 13 PKTC that continued on to transplant. One patient experienced antibody mediated rejection that did not correlate to any pre-formed or de novo anti-HLA-Ab that emerged post vaccinations.
*Conclusions: Vaccination resulted in a significant, and transient, increase in median MFI from baseline to 3-4 week follow-up that decreased by 7-10 week follow-up. The majority of responses were non-specific, hypothesized to be related to denatured antigens on single antigen beads. Five confirmed de novo anti-HLA Ab were detected and deemed secondary to vaccination. These data suggest limited clinical impact of vaccinations on emergence of de novo anti-HLA Ab.
To cite this abstract in AMA style:
Sweiss H, Lyons J, Hall R, Hitchman K, Ranch D, Kincaide E, Crowther B. Impact of Catch-Up Vaccinations on Anti-HLA Antibody Responses in Pediatric Kidney Transplant Candidates [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-catch-up-vaccinations-on-anti-hla-antibody-responses-in-pediatric-kidney-transplant-candidates/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress