Impact of a Cmv Cell Mediated Immunity Based Protocol on Guiding Cmv Prophylaxis Following Pediatric Liver Transplantation – A Single Center Experience
G. Kalkan1, S. Ball2, L. McConnell2, D. M. Desai3, A. Aqul3, P. K. Sue1
1Infectious Disease, University of Texas Southwestern Medical Center, Dallas, TX, 2Solid Organ Transplant Program, Children's Health, Dallas, TX, 3Solid Organ Transplant Program, University of Texas Southwestern Medical Center, Dallas, TX
Meeting: 2021 American Transplant Congress
Abstract number: 228
Keywords: Cytomeglovirus, Liver transplantation, Outcome, Prophylaxis
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: Infectious Disease Poutpouri
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:45pm-4:50pm
Presentation Time: 4:45pm-4:50pm
Location: Virtual
*Purpose: Cytomegalovirus (CMV) is an important cause of morbidity following pediatric orthotopic liver transplantation (OLT), especially among high risk (HR) CMV D+/R- recipients. In 2019, our institution adopted a Cell Mediated Immunity (CMI) based hybrid approach to guide CMV prophylaxis following pediatric OLT. We report our experience using this approach and its implication on the rate of CMV infection, rejection, and valganciclovir (VGC) adverse reactions and cost in our patient population.
*Methods: Initial protocol required patients to remain on prophylactic VGC until a CMV IgG is detected (cohort 1). Modified protocol (cohort 2) stratified patients into 3 risk groups; Low (LR) remains on VGC until 6 months post-transplant, intermediate (IR), and high (HR) utilizes CMV CMI to guide VGC prophylaxis duration. Retrospective review of pediatric OLT recipients between January 2017 – December 2018 (cohort 1) and January 2019 – November 2020 (cohort 2). Patients characteristics, CMV infection, CMV disease, rejection, duration of valganciclovir (VGC) prophylaxis, cost of VGC, and drug attributable adverse events were compared between the two cohorts. Fischer’s exact test and student T-Test were used to assess differences in categorical and ordinal outcomes between the two cohorts, respectively.
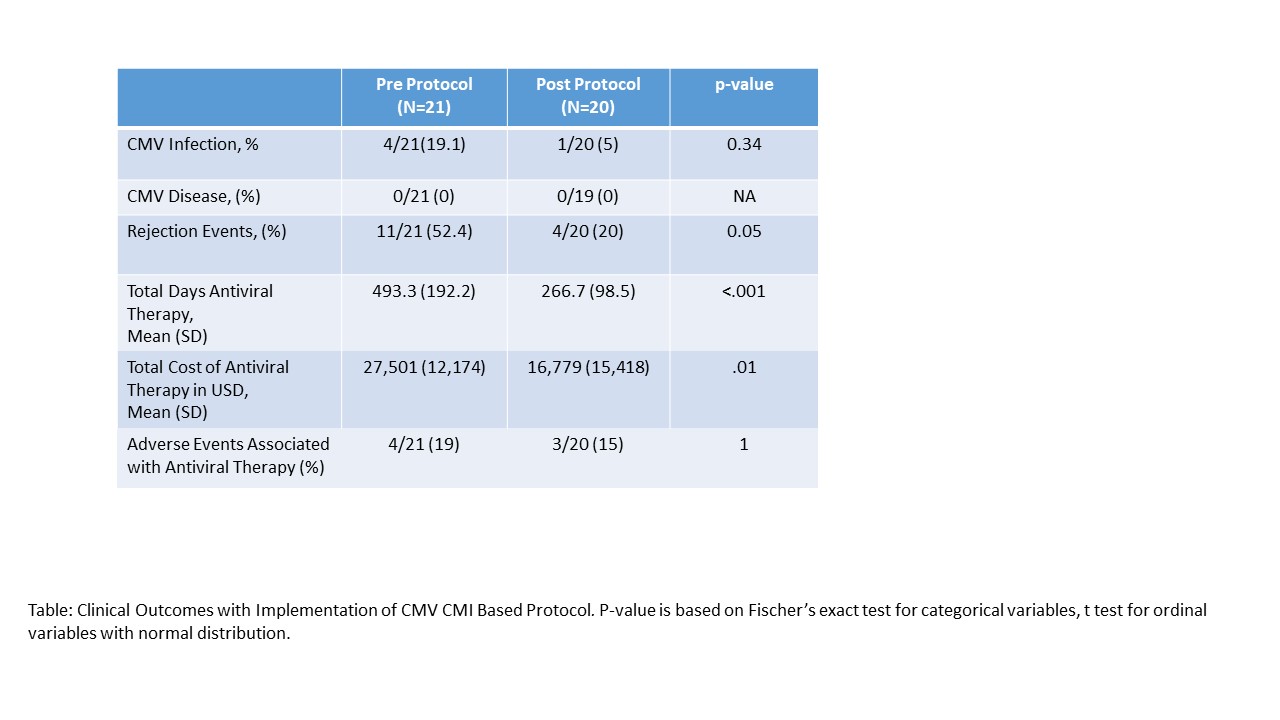
*Results: 41 children (21 in cohort 1, 20 in cohort 2), Eight (19.5%) were considered HR (D+/R-), 21 (51.2%) were intermediate (IR) (D+/R+), and 12 (29.2%) were low (D-/R-) risk for CMV infection. All received VGC prophylaxis. Cohort 2 demonstrated no increase in the incidence of CMV infection or disease and decreased episodes of rejection compared to cohort 1. Mean duration of antiviral treatment was decreased by 41%, and associated with a decrease in cost of antivirals by 52%. Adverse events associated with VGC were similar in both groups 19 vs 15%.
*Conclusions: Implementation of a CMV CMI based protocol decreased the cost and total days of antiviral prophylaxis following pediatric OLT, and was not associated with increased risk of breakthrough viremia or CMV disease. Incorporating CMV specific CMI may be a useful tool to guide post-transplant CMV prophylaxis and reduce unnecessary antiviral use.
To cite this abstract in AMA style:
Kalkan G, Ball S, McConnell L, Desai DM, Aqul A, Sue PK. Impact of a Cmv Cell Mediated Immunity Based Protocol on Guiding Cmv Prophylaxis Following Pediatric Liver Transplantation – A Single Center Experience [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-a-cmv-cell-mediated-immunity-based-protocol-on-guiding-cmv-prophylaxis-following-pediatric-liver-transplantation-a-single-center-experience/. Accessed July 1, 2025.« Back to 2021 American Transplant Congress