Immunogenic HLA Mismatches and Evidence That Belatacept May Be the First Therapy Able to Prevent DQ De Novo DSA: An Analysis of the BENEFIT and BENEFIT-EXT Trial Cohorts
1Terasaki Research Institute, Los Angeles, CA
2Immunology Biomarker Group, Bristol-Myers Squibb, Princeton, NJ
3Pathology & Laboratory Medicine, Emory University School of Medicine, Atlanta, GA.
Meeting: 2018 American Transplant Congress
Abstract number: C43
Keywords: Efficacy, HLA antibodies, Immunogenicity, Kidney transplantation
Session Information
Session Name: Poster Session C: Kidney Donor Selection / Management Issues
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
The use of belatacept over cyclosporine is associated with lower rates of DSA development in adult kidney transplantation. However, the effect of belatacept on the immunogenicity of individual HLA mismatches (MM) remains to be elucidated.
We studied the immunogenicity, defined as the development of de novo DSA (dnDSA), of HLA-A, -B, -C, -DR and -DQ MM (serological) in a cohort of 870 kidney transplant patients from the BENEFIT and BENEFIT-EXT trials, who received cyclosporine (n=291) or belatacept (n=579) as first-line immunosuppression.
A total of 3339 MM were studied; the MM in the cyclosporine group (n=1129) were significantly more immunogenic than MM in the belatacept group (n=2210) for all HLA loci studied (p<0.0001, Figure 1A). The immunogenicity of DQ MM was the one most impacted by belatacept treatment, where only 3% of MM were immunogenic compared to 23% in the cyclosporine group (p<0.0001). Furthermore, the highly immunogenic MM at DQ5 and DQ7 were significantly inhibited by belatacept treatment (p=0.011 and p=0.019, respectively) (Figure 1B). Among HLA class I MM, only A2, A24 and B45 were significantly inhibited by belatacept treatment. The immunogenicity of all other MM was not significantly inhibited by belatacept, although a trend was evident, particularly for HLA-C MM.
The use of belatacept, compared to cyclosporine, as first-line immunosuppression reduced the development of dnDSA, particularly for DQ dnDSA. Belatacept immunosuppression should be considered for HLA-DQ mismatched transplant patients. Belatacept is the first therapy to demonstrate the ability to prevent formation of DQ dnDSA. 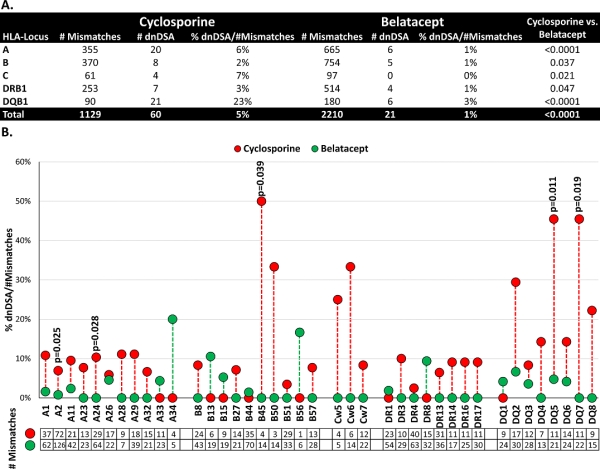
CITATION INFORMATION: Everly M., Jucaud V., Roberts M., Gebel H., Bray R. Immunogenic HLA Mismatches and Evidence That Belatacept May Be the First Therapy Able to Prevent DQ De Novo DSA: An Analysis of the BENEFIT and BENEFIT-EXT Trial Cohorts Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Everly M, Jucaud V, Roberts M, Gebel H, Bray R. Immunogenic HLA Mismatches and Evidence That Belatacept May Be the First Therapy Able to Prevent DQ De Novo DSA: An Analysis of the BENEFIT and BENEFIT-EXT Trial Cohorts [abstract]. https://atcmeetingabstracts.com/abstract/immunogenic-hla-mismatches-and-evidence-that-belatacept-may-be-the-first-therapy-able-to-prevent-dq-de-novo-dsa-an-analysis-of-the-benefit-and-benefit-ext-trial-cohorts/. Accessed February 24, 2026.« Back to 2018 American Transplant Congress
