Human PBMC-Transferred Murine MHC Class I/II-Deficient NOG Mice Enable Long-Term Evaluation of Human Immune Responses to Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets
Center for Immune Therapeutics and Diagnosis, Juntendo University, Tokyo, Japan
Meeting: 2022 American Transplant Congress
Abstract number: 182
Keywords: Image analysis, Immunogenicity, Stem cells, T cell graft infiltration
Topic: Basic Science » Basic Science » 06 - Tissue Engineering and Regenerative Medicine
Session Information
Session Name: Cellular/Islet Therapies and Tissue Engineering
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:30pm-6:40pm
Presentation Time: 6:30pm-6:40pm
Location: Hynes Room 310
*Purpose: Transplantation of cardiomyocytes sheets derived from allogenic human induced pluripotent stem cells (hiPS-CMs) hold great promise in generating new functional myocardium in situ. However, immunogenicity of hiPS-CMs has not yet been fully investigated because previous experiment models have limitations in functional T cell development and long-term human T cells engraftment without severe graft-versus-host-disease (GVHD). The purpose of this study is to establish a new humanized mouse model to evaluate the immunogenicity of hiPS-CMs in the long term.
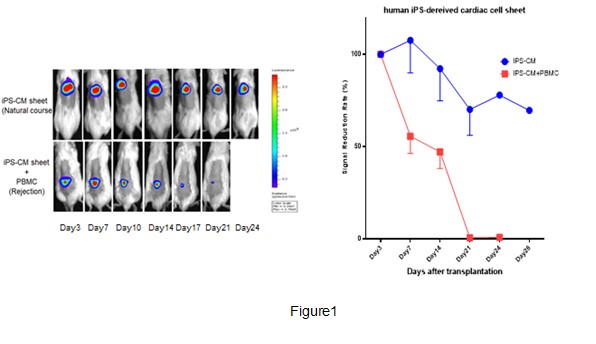
*Methods: We generated luciferase transduced allogenic hiPS-CMs (lu-hiPS-CMs), representing the survival of hiPS-CMs. We transferred hPBMC (10 million) to murine MHC class I/II deficient NOG mice (NOG-dKO; NOG-b2m, IAb double KO mouse) intravenously. The engraftment of human T cells in the peripheral blood in NOG-dKO with hPBMC was monitored by flow cytometry over time. The two lu-hiPS-CMs (10 million/sheet) were overlapped and transplanted into the subcutaneous space of NOG-dKO with or without hPBMC. The graft survival was monitored via serial bioluminescent image (BLI) over time by using the Xenogen In Vivo Imaging System. Each BLI was evaluated on the basis of that of Day 3 (Signal reduction rate). The symptoms of diarrhea and weight loss susceptive of GVHD were also evaluated after hPBMC transfer.
*Results: The engraftment of human T cell was preserved more than 90 days in NOG-dKO with hPBMC. BLI of hiPS-CMs in NOG-dKO with hPBMC was dramatically reduced compared to that of NOG-dKO without hPBMC, and completely disappeared at Day21. The histopathology of transplanted area showed the abundant human CD8+ T cells infiltration along the hiPS-CM cells (Figure1). Moreover, BLI of hiPS-CMs in NOG-dKO with CD8+ T cells depleted hPBMC showed the milder reduction than that of NOG-dKO with hPBMC. NOG-dKO with hPBMC did not exhibit any signs of GVHD.
*Conclusions: Humanized mouse model using NOG-dKO counts as a promising tool to analyze the human immune response specific to hiPS-CMs, enabling the appropriate immunosuppressive strategies of hiPS-CMs in the further study.
To cite this abstract in AMA style:
Matsumoto R. Human PBMC-Transferred Murine MHC Class I/II-Deficient NOG Mice Enable Long-Term Evaluation of Human Immune Responses to Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/human-pbmc-transferred-murine-mhc-class-i-ii-deficient-nog-mice-enable-long-term-evaluation-of-human-immune-responses-to-human-induced-pluripotent-stem-cell-derived-cardiomyocyte-sheets/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress