First-in-human Study Of Lis1, A Novel Glyco-humanized Anti-lymphocyte Swine Polyclonal Antibody, In Kidney Transplantation: Safety And Efficacy
1Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Xenothera, Nantes, France, 3Laboratorio di Tecnologie della Riproduzione, Cremone, Italy, 4IECM, Immunoendocrinology, USC1383, Oniris, INRAE, Nantes, France, 5Transplantation Immunology Unit, Padua University Hospital, Padova, Italy, 6Nantes Universités, Nantes, France
Meeting: 2022 American Transplant Congress
Abstract number: 9073
Keywords: Antilymphocyte antibodies, Humanized antibodies, Immunogenicity, Kidney transplantation
Topic: Clinical Science » Kidney » 37 - Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: to assess safety, T cell depletion, PK/PD and determine the therapeutic dose of LIS1, a purified anti-lymphocyte glyco-humanized polyclonal swine IgG, derived by immunizing with a human T cell line double Knock-Out swine lacking the two xeno-antigens α-1-3 galactose (αGal) and N-glycolylneuraminic acid (Neu5Gc), thus avoiding serum sickness and the possible systemic inflammation called xenosialitis, LIS1 is “FC-silenced” so that it mediates no antibody-dependent cell-mediated cytotoxicity but strongly activates complement-mediated cytotoxicity. Preclinical studies in non-human primates demonstrated (i) selective depletion of T cells (CD4+ and CD8+) versus myeloid, Treg and B cells, this depletion being more controlled in time as compared to rabbit ALG, and (ii) prevention of skin graft rejection similarly to rabbit ALG.
*Methods: phase Ib, open-label, single site, conducted in two sequential cohorts, Ascending Dose cohort (n=5) and Therapeutic Dose cohort (n=5). LIS1 administered by IV central catheter over 8-12 hours, one daily dose, 5 consecutive days, starting at day 1 post-transplantation, in first kidney transplant patients with PRA < 50%, FCXM-, DSA, EBV+.
*Results: 10 patients were included, 6 men and 4 women, mean PRA 7.8 % (± 12.3), mean age 48,6 years (± 8,7). All received tacrolimus, mycophenolic acid, and steroids, as maintenance therapy. Safety: No platelets depletion, nor related adverse event were observed.
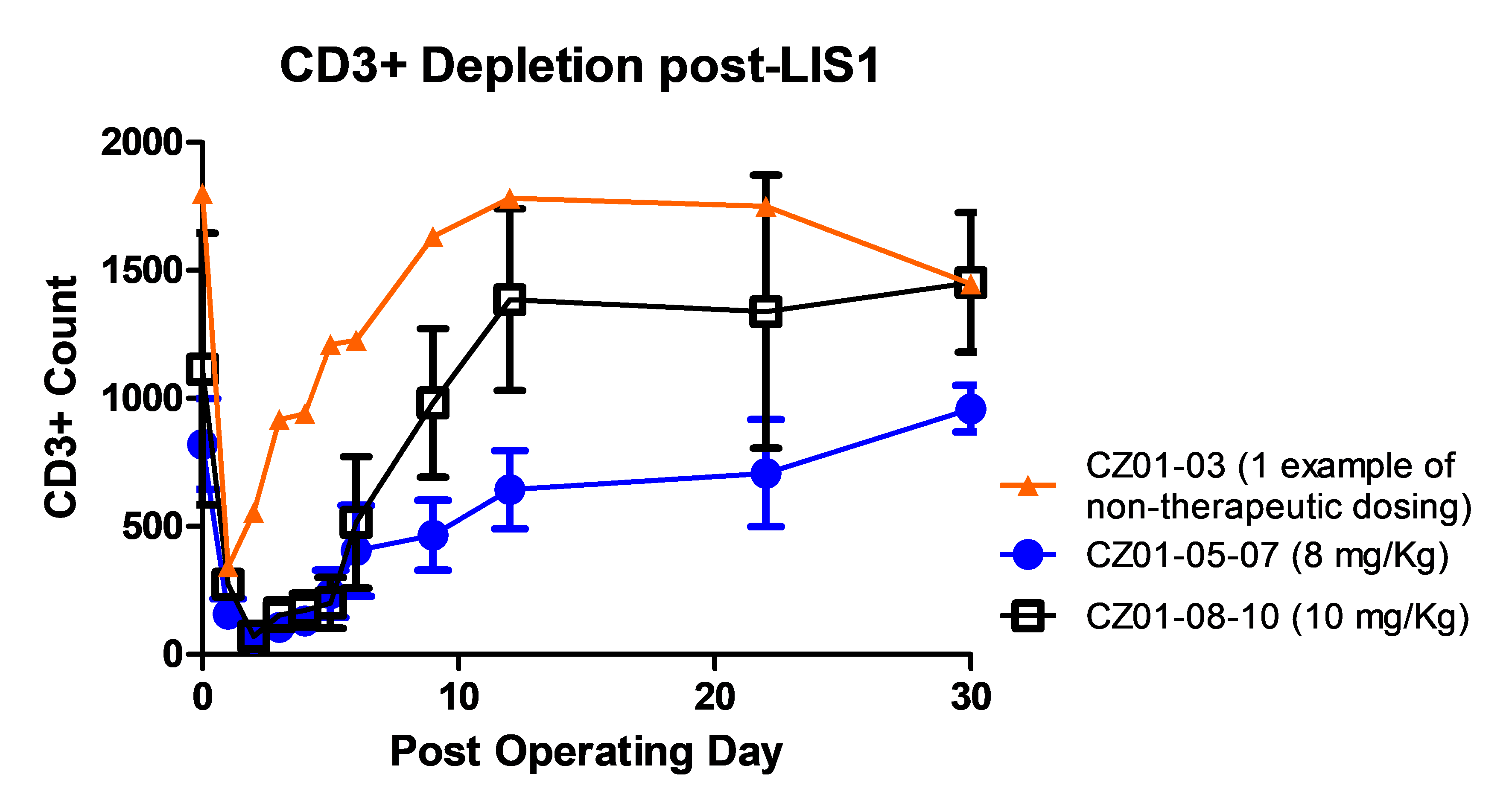
Efficacy:Doses of 0.6; 1 and 3 mg/Kg were suboptimal. Dose of 6 mg/kg resulted in CD3+ T-cell depletion < 100/mm3 at day 2 with recovery within a week. Doses of 8 and 10 mg/Kg resulted in CD3+ T-cell depletion < 100/mm3 at day 2 with recovery within 2 to 4 weeks (see figure).
*Conclusions: LIS1 at a dose of 8-10 mg/kg is safe and well tolerated and allows a controlled-in-time selective depletion of target CD3+ T cells. Phase II multicentric double blind study is planned.
To cite this abstract in AMA style:
Slatinska J, Vanhove B, Kolarova M, Shneiker F, Rousse J, Galli C, Bach J, Cozzi E, Soulillou J, Duvaux O, Viklicky O. First-in-human Study Of Lis1, A Novel Glyco-humanized Anti-lymphocyte Swine Polyclonal Antibody, In Kidney Transplantation: Safety And Efficacy [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/first-in-human-study-of-lis1-a-novel-glyco-humanized-anti-lymphocyte-swine-polyclonal-antibody-in-kidney-transplantation-safety-and-efficacy/. Accessed July 4, 2025.« Back to 2022 American Transplant Congress